Study on efficient arsenic removal performance and mechanism of natural ferromanganese ore
-
摘要:
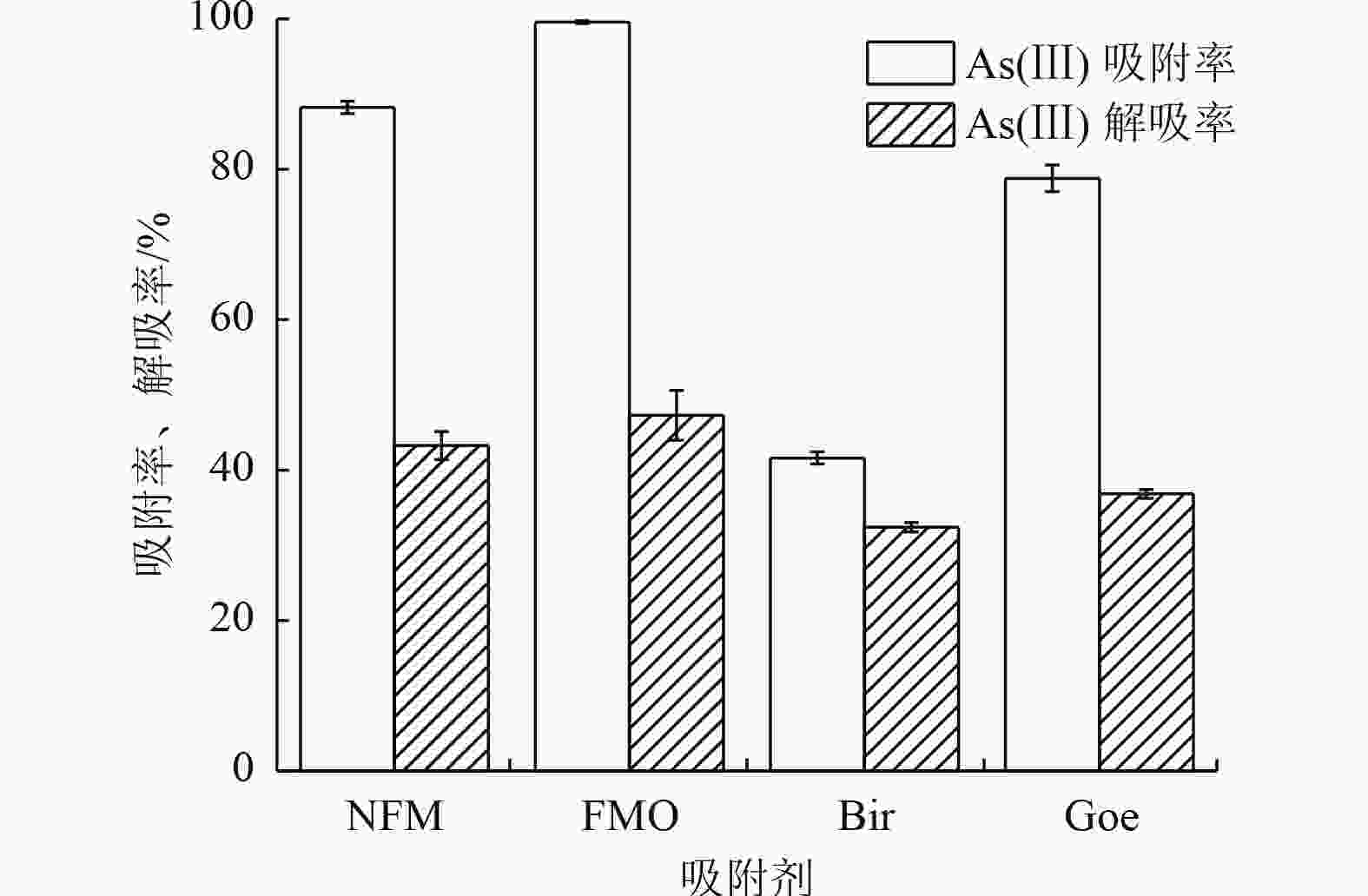
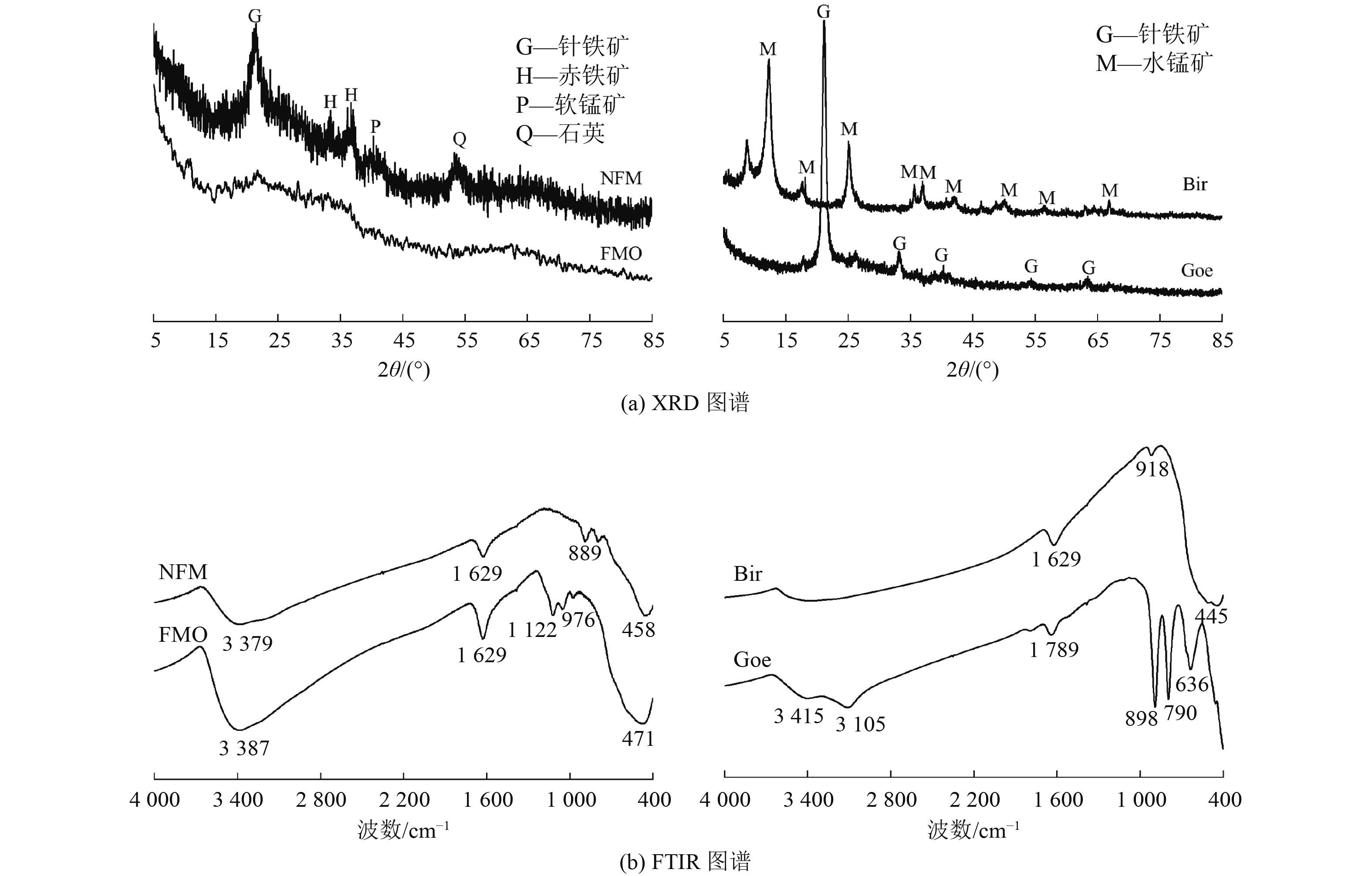
为开发高效、廉价的水体As(Ⅲ)去除材料,采用天然铁锰矿(NFM)作为吸附剂,通过动力学、热力学、等温吸附以及吸附/解吸试验评估其对As(Ⅲ)的吸附性能,结合傅里叶变换红外光谱、扫描电镜以及X射线光电子能谱(XPS)等表征手段进行机理分析,并与铁锰二元氧化物(FMO)、水钠锰矿(Bir)和针铁矿(Goe)的吸附特性进行对比。结果表明:NFM主要由锰氧化物和铁氧化物组成,铁锰摩尔比为6∶1,比表面积为280.4 m2/g,对As(Ⅲ)的饱和吸附容量为48.3 mg/g。Freundlich模型和准二级动力学模型能较好地拟合NFM的吸附过程。XPS等分析表明,NFM的吸附和氧化的协同作用是去除As(Ⅲ)的关键因素。其中,锰氧化物展示出优异的氧化As(Ⅲ)的能力,而铁氧化物具有强的吸附作用。
Abstract:In order to develop an efficient and inexpensive material for As(Ⅲ) removal from water, natural ferromanganese ore (NFM) was used as adsorbent. Kinetic, thermodynamic, isothermal adsorption and adsorption/desorption experiments were conducted to evaluate the adsorption performance of As(Ⅲ). The mechanism was analyzed by Fourier transform infrared spectroscopy, scanning electron microscopy, X-ray photoelectron spectroscopy, and the adsorption characteristics were compared with those of iron-manganese binary oxide (FMO), birnessite (Bir), and goethite (Goe). The results showed that NFM was mainly composed of manganese oxide and iron oxide, with a Fe-Mn molar ratio of 6∶1, the specific surface area of 280.4 m2/g, and a saturation adsorption capacity of 48.3 mg/g for As(Ⅲ). The Freundlich model and the pseudo-second order kinetic model could better fit the adsorption process of NFM. XPS and other characterization analyses indicated that the synergistic effect of adsorption and oxidation of NFM was the key factor for As(Ⅲ) removal. Among them, manganese oxides exhibited excellent oxidation of As(Ⅲ), while iron oxides had strong adsorption.
-
Key words:
- As(Ⅲ) /
- natural ferromanganese ore /
- manganese oxide /
- iron oxide /
- adsorption /
- oxidation
-
表 1 NFM元素含量分析
Table 1. Percentage content of each element of NFM
% 元素 含量 元素 含量 Fe 72.32 P 0.76 Mn 11.78 Zn 0.09 Ca 0.33 Ni 0.04 Si 3.46 As 0.01 Al 2.52 Pb 0.02 Mg 0.27 Cr 0.01 注:仅显示含量大于0.01%的元素。 表 2 吸附剂的Fe、Mn含量和比表面积
Table 2. Fe, Mn content and specific surface area of the adsorbent
吸附剂 元素含量/% 铁锰比 比表面积/(m2/g) Fe Mn NFM 72.3 11.8 1∶6.12 280.4 FMO 46.9 13.5 3.47∶1 268.8 Bir 52.3 102.9 Goe 67.4 95.8 表 3 吸附剂对As(Ⅲ)的吸附的Langmuir和Freundlich模型参数
Table 3. Langmuir and Freundlich model parameters for adsorption of As(Ⅲ) on adsorbents
吸附剂 Langmuir模型 Freundlich模型 qm b R2 Kf n R2 NFM 48.3 0.0649 0.962 8.07 3.36 0.998 FMO 70.9 0.350 0 0.973 17.4 3.09 0.957 Bir 33.2 0.006 0 0.662 0.86 1.91 0.977 Goe 44.3 0.038 0 0.905 5.81 3.04 0.984 表 4 不同温度下As(Ⅲ)在吸附剂上吸附的热力学参数
Table 4. Thermodynamic parameters of As(Ⅲ) adsorption on adsorbents at different temperatures
吸附剂 ΔG0/(kJ/mol) ΔH0
/
(kJ/mol)ΔS0
/
〔J/(K·mol)〕R2 278 K 298 K 308 K NFM −41.0 −44.0 −45.4 27.7 0.0148 0.9999 FMO −16.6 −20.9 −23.1 44.2 0.218 0 0.8714 Bir −8.71 −9.42 −9.77 1.18 0.0356 0.9899 Goe −12.4 −13.6 −14.2 4.55 0.061 0 0.8936 表 5 准一级动力学模型和准二级动力学模型拟合参数
Table 5. Fitting parameters of pseudo-first-order and pseudo-second-order kinetic models
吸附剂 准一级动力学模型 准二级动力学模型 qe1 K1 R2 qe2 K2 h0 R2 NFM 8.22 0.124 0.983 21.9 0.0563 27.0 0.998 6 FMO 5.68 0.196 0.966 23.9 0.153 87.4 0.999 9 Bir 1.14 0.068 9 0.205 7.07 0.559 27.9 0.998 5 Goe 4.23 0.187 0.955 17.6 0.175 54.2 0.999 7 表 6 吸附剂中不同价态Mn、Fe、 As的原子占比
Table 6. Atomic percentages of the different valence of Mn, Fe, As in absorbents
% 吸附剂 Mn(Ⅱ) Mn(Ⅲ) Mn(Ⅳ) Fe(Ⅱ) Fe(Ⅲ) As(Ⅲ) As(Ⅴ) NFM 19.64 59.53 20.83 36.07 63.93 0 0 NFM-As(Ⅲ) 62.89 21.39 15.72 53.27 46.73 47.78 52.22 FMO 11.48 24.64 63.88 63.03 36.97 0 0 FMO-As(Ⅲ) 60.28 19.44 20.28 62.42 37.58 29.39 70.61 Bir 54.38 20.84 24.78 0 0 0 0.00 Bir-As(Ⅲ) 62.22 13.42 24.36 0 0 35.50 64.50 Goe 0 0 0 63.38 36.62 0 0 Goe-As(Ⅲ) 0 0 0 49.13 50.87 56.54 43.46 -
[1] NORDSTROM D K. Worldwide occurrences of arsenic in ground water[J]. Science,2002,296:2143-2145. doi: 10.1126/science.1072375 [2] JOMOVA K, JENISOVA Z, FESZTEROVA M, et al. Arsenic: toxicity, oxidative stress and human disease[J]. Journal of Applied Toxicology,2011,31(2):95-107. [3] GALAL G H, OZOLINS G. WHO guidelines for drinking-water quality[J]. Water Supply,1993,11(3):1-16. [4] SMEDLEY P L, KINNIBURGH D G. A review of the source, behaviour and distribution of arsenic in natural waters[J]. Applied Geochemistry,2002,17(5):517-568. doi: 10.1016/S0883-2927(02)00018-5 [5] MENG X, JING C, KORFIATIS G P. A review of redox transformation of arsenic in aquatic environments[J]. ACS Symposium Series,2003,835:70-83. [6] 张元元, 郭少娟, 王菲菲,等.TCDD和汞, 镉, 铅, 砷联合毒性效应及机理研究进展[J]. 环境工程技术学报,2021,11(2):332-342. doi: 10.12153/j.issn.1674-991X.20200217ZHANG Y Y, GUO S J, WANG F F, et al. Research progress on joint toxic effects and mechanisms of the mixture of TCDD and mercury, cadmium, lead, arsenic[J]. Journal of Environmental Engineering Technology,2021,11(2):332-342. doi: 10.12153/j.issn.1674-991X.20200217 [7] LIANG M, GUO H, XIU W. Mechanisms of arsenite oxidation and arsenate adsorption by a poorly crystalline manganese oxide in the presence of low molecular weight organic acids[C]//E3S Web of Conferences. France: EDP Sciences, 2019: 04009. [8] LIANG M, GUO H, XIU W. Arsenite oxidation and arsenic adsorption on birnessite in the absence and the presence of citrate or EDTA[J]. Environmental Science and Pollution Research,2020,27(35):43769-43785. doi: 10.1007/s11356-020-10292-3 [9] HOU J, TAN X, XIANG Y, et al. Insights into the underlying effect of Fe vacancy defects on the adsorption affinity of goethite for arsenic immobilization[J]. Environmental Pollution,2022,314:120268. doi: 10.1016/j.envpol.2022.120268 [10] CUONG D V, WU P C, CHEN L I, et al. Active MnO2/biochar composite for efficient As(Ⅲ) removal: insight into the mechanisms of redox transformation and adsorption[J]. Water Research,2021,188:116495. doi: 10.1016/j.watres.2020.116495 [11] 谷倩, 张琢, 张丽,等.砷污染场地土壤的稳定化技术工程应用研究[J]. 环境工程技术学报,2021,11(4):734-739. doi: 10.12153/j.issn.1674-991X.20200203GU Q, ZHANG Z, ZHANG L, et al. Research on engineering application of stabilization technology for arsenic contaminated site soil[J]. Journal of Environmental Engineering Technology,2021,11(4):734-739. doi: 10.12153/j.issn.1674-991X.20200203 [12] SU W, XIAO L. Manganese-doped ferrihydrite/cellulose/polyvinyl alcohol composite membrane: easily recyclable adsorbent for simultaneous removal of arsenic and cadmium from soil[J]. Science of the Total Environment,2022,815:152748. doi: 10.1016/j.scitotenv.2021.152748 [13] 石乐琪, 郭莉, 吕晨阳等.地下水脱砷技术的研究现状及发展趋势[J]. 环境工程技术学报,2022,12(5):1548-1554. doi: 10.12153/j.issn.1674-991X.20210284SHI L Q, GUO L, LU C Y, et al. Research status and development trend of the technology for arsenic removal from groundwater[J]. Journal of Environmental Engineering Technology,2022,12(5):1548-1554. doi: 10.12153/j.issn.1674-991X.20210284 [14] YIN C, LI S, LIU L, et al. Structure-tunable trivalent Fe-Al-based bimetallic organic frameworks for arsenic removal from contaminated water[J]. Journal of Molecular Liquids,2022,346:117101. doi: 10.1016/j.molliq.2021.117101 [15] ZHENG Q, HOU J, HARTLEY W, et al. As(Ⅲ) adsorption on Fe-Mn binary oxides: are Fe and Mn oxides synergistic or antagonistic for arsenic removal[J]. Chemical Engineering Journal,2020,389:124470. doi: 10.1016/j.cej.2020.124470 [16] LIN Y, JIN X, KHAN N I, et al. Bimetallic Fe/Ni nanoparticles derived from green synthesis for the removal of arsenic(Ⅴ) in mine wastewater[J]. Journal of Environmental Management,2022,301:113838. doi: 10.1016/j.jenvman.2021.113838 [17] BAI Y, YANG T, LIANG J, et al. The role of biogenic Fe-Mn oxides formed in situ for arsenic oxidation and adsorption in aquatic ecosystems[J]. Water Research,2016,98:119-127. doi: 10.1016/j.watres.2016.03.068 [18] CHEN D, LI D, XIAO Z, et al. Removal of lead ions by two Fe-Mn oxide substrate adsorbents[J]. Science of the Total Environment,2021,773:145670. doi: 10.1016/j.scitotenv.2021.145670 [19] PARSONS J G, LOPEZ M L, PERALTA J R, et al. Determination of arsenic(Ⅲ) and arsenic(Ⅴ) binding to microwave assisted hydrothermal synthetically prepared Fe3O4, Mn3O4, and MnFe2O4 nanoadsorbents[J]. Microchemical Journal,2009,91(1):100-106. doi: 10.1016/j.microc.2008.08.012 [20] ZHANG G S, QU J H, LIU H J, et al. Removal mechanism of As(Ⅲ) by a novel Fe-Mn binary oxide adsorbent: oxidation and sorption[J]. Environmental Science and Technology,2007,41(13):4613-4619. doi: 10.1021/es063010u [21] MCKENZIE R M. The synthesis of birnessite, cryptomelane, and some other oxides and hydroxides of manganese[J]. Mineralogical Magazine,1971,38:493-502. doi: 10.1180/minmag.1971.038.296.12 [22] ATKINSON R J, POSNER A M, QUIRK J P. Adsorption of potential-determining ions at the ferric oxide-aqueous electrolyte interface[J]. Journal of Physical Chemistry,1967,71(3):550-558. doi: 10.1021/j100862a014 [23] WANG J, GUO X. Adsorption isotherm models: classification, physical meaning, application and solving method[J]. Chemosphere,2020,258:127279. doi: 10.1016/j.chemosphere.2020.127279 [24] FOO K Y, HAMEED B H. Insights into the modeling of adsorption isotherm systems[J]. Chemical Engineering Journal,2010,156(1):2-10. doi: 10.1016/j.cej.2009.09.013 [25] QI J, ZHANG G, LI H. Efficient removal of arsenic from water using a granular adsorbent: Fe-Mn binary oxide impregnated chitosan bead[J]. Bioresource Technology,2015,193:243-249. doi: 10.1016/j.biortech.2015.06.102 [26] CAI G, TIAN Y, LI D, et al. Self-enhanced and efficient removal of As(Ⅲ) from water using Fe-Cu-Mn composite oxide under visible-light irradiation: synergistic oxidation and mechanisms[J]. Journal of Hazardous Materials,2022,422:126908. doi: 10.1016/j.jhazmat.2021.126908 [27] LOU Z, CAO Z, XU J, et al. Enhanced removal of As(Ⅲ)/(Ⅴ) from water by simultaneously supported and stabilized Fe-Mn binary oxide nanohybrids[J]. Chemical Engineering Journal,2017,322:710-721. doi: 10.1016/j.cej.2017.04.079 [28] ALLARD S, GUTIERREZ L, FONTAINE C, et al. Organic matter interactions with natural manganese oxide and synthetic birnessite[J]. Science of the Total Environment,2017,583:487-495. doi: 10.1016/j.scitotenv.2017.01.120 [29] CHENG Z, FU F, DIONYSIOU D D, et al. Adsorption, oxidation, and reduction behavior of arsenic in the removal of aqueous As(Ⅲ) by mesoporous Fe/Al bimetallic particles[J]. Water Research,2016,96:22-31. doi: 10.1016/j.watres.2016.03.020 [30] BAI Y, TANG X, SUN L, et al. Application of iron-based materials for removal of antimony and arsenic from water: sorption properties and mechanism insights[J]. Chemical Engineering Journal,2021,431:134143. [31] ZHENG Q, TU S, HOU J, et al. Insights into the underlying mechanisms of stability working for As(Ⅲ) removal by Fe-Mn binary oxide as a highly efficient adsorbent[J]. Water Research,2021,203:117558. doi: 10.1016/j.watres.2021.117558 [32] YIN H, LIU F, FENG X, et al. Co2+-exchange mechanism of birnessite and its application for the removal of Pb2+ and As(Ⅲ)[J]. Journal of Hazardous Materials,2011,196:318-326. doi: 10.1016/j.jhazmat.2011.09.027 [33] 蔡金水, 康得军, 杨天学,等.铁改性杭锦土吸附剂对水中砷的去除研究[J]. 环境科学研究,2021,34(2):346-355.CAI J S, KANG D J, YANG T X et al. Removal of arsenic from water by iron modified Hangjin clay adsorbent[J]. Research of Environmental Sciences,2021,34(2):346-355. [34] JOSHI T P, ZHANG G, JEFFERSON W A, et al. Adsorption of aromatic organoarsenic compounds by ferric and manganese binary oxide and description of the associated mechanism[J]. Chemical Engineering Journal,2017,309:577-587. doi: 10.1016/j.cej.2016.10.084 [35] YU X, WEI Y, LIU C, et al. Ultrafast and deep removal of arsenic in high-concentration wastewater: a superior bulk adsorbent of porous Fe2O3 nanocubes-impregnated graphene aerogel[J]. Chemosphere,2019,222:258-266. doi: 10.1016/j.chemosphere.2019.01.130 [36] ZHANG G, XU X, JI Q, et al. Porous nanobimetallic Fe-Mn cubes with high valent Mn and highly efficient removal of arsenic(Ⅲ)[J]. ACS Applied Materials and Interfaces,2017,9(17):14868-14877. doi: 10.1021/acsami.7b02127 [37] SHUMLAS S L, SINGIREDDY S, THENUWARA A C, et al. Oxidation of arsenite to arsenate on birnessite in the presence of light[J]. Geochemical Transactions,2016,17(1):1-10. doi: 10.1186/s12932-016-0033-9 [38] JAISWAL A, BANERJEE S, MANI R, et al. Synthesis, characterization and application of goethite mineral as an adsorbent[J]. Journal of Environmental Chemical Engineering,2013,1(3):281-289. doi: 10.1016/j.jece.2013.05.007 [39] PARK J H, HAN Y S, AHN J S. Comparison of arsenic co-precipitation and adsorption by iron minerals and the mechanism of arsenic natural attenuation in a mine stream[J]. Water Research,2016,106:295-303. doi: 10.1016/j.watres.2016.10.006 [40] XIONG Y, TONG Q, SHAN W, et al. Arsenic transformation and adsorption by iron hydroxide/manganese dioxide doped straw activated carbon[J]. Applied Surface Science,2017,416:618-627. doi: 10.1016/j.apsusc.2017.04.145 [41] 许江城, 康得军, 赵颖,等.高效除砷分子筛新型材料制备及其吸附特性研究[J]. 环境科学研究,2020,33(9):2191-2201.XU J C, KANG D J, ZHAO Y, et al. Preparation and adsorption characteristics of novel molecular sieve for high sufficiency arsenic removal[J]. Research of Environmental Sciences,2020,33(9):2191-2201. [42] HU Q, LIU Y, GU X, et al. Adsorption behavior and mechanism of different arsenic species on mesoporous MnFe2O4 magnetic nanoparticles[J]. Chemosphere,2017,181:328-336. doi: 10.1016/j.chemosphere.2017.04.049 [43] XU W H, WANG L, WANG J, et al. Superparamagnetic mesoporous ferrite nanocrystal clusters for efficient removal of arsenite from water[J]. CrystEngComm,2013,15(39):7895-7903. doi: 10.1039/c3ce40944a [44] ZHU M, PAUL K W, KUBICKI J D, et al. Quantum chemical study of arsenic(Ⅲ, Ⅴ) adsorption on Mn-oxides: Implications for arsenic(Ⅲ) oxidation[J]. Environmental Science and Technology,2009,43(17):6655-6661. doi: 10.1021/es900537e [45] MCCANN C M, PEACOCK C L, HUDSON-EDWARDS K A, et al. In situ arsenic oxidation and sorption by a Fe-Mn binary oxide waste in soil[J]. Journal of Hazardous Materials,2018,342:724-731. doi: 10.1016/j.jhazmat.2017.08.066 [46] ZHANG G, LIU F, LIU H, et al. Respective role of Fe and Mn oxide contents for arsenic sorption in iron and manganese binary oxide: an X-ray absorption spectroscopy investigation[J]. Environmental Science and Technology,2014,48(17):10316-10322. ⊗ doi: 10.1021/es501527c -





 下载:
下载: