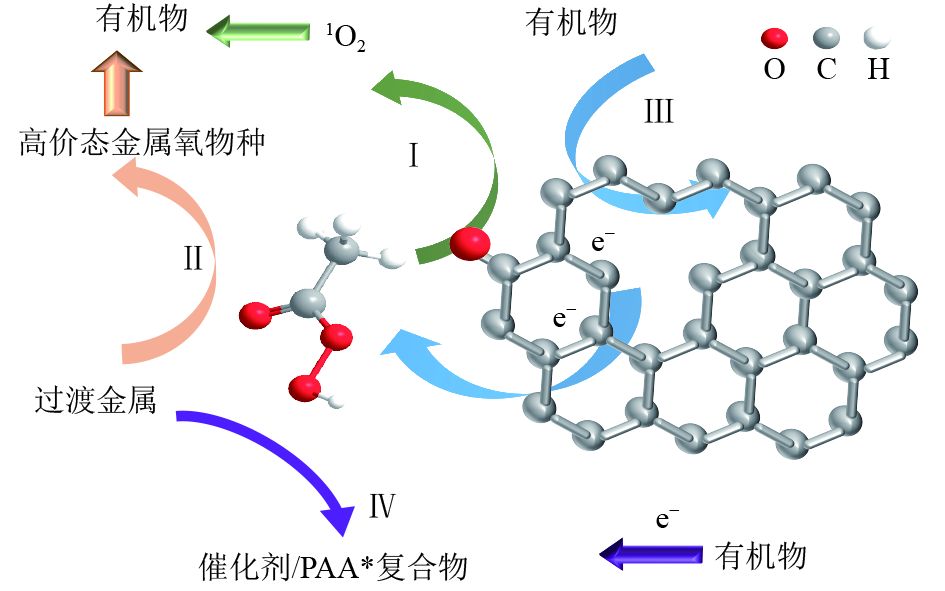
Research progress on the degradation of environmental organic pollutants by activated peracetic acid technology
-
摘要:
近年来,以抗生素、内分泌干扰物等为代表的新污染物在环境中被频繁检出,对生态系统和人类健康构成潜在风险,高效稳定的有机污染物控制技术研发是当前环境领域的研究热点。以活化过氧乙酸的高级氧化技术为研究对象,对过渡金属、碳材料及其复合材料活化过氧乙酸的效果及其降解有机污染物的机制进行系统论述,重点探讨反应过程中的自由基(有机自由基、羟基自由基)和非自由基(单线态氧、高价金属氧物种、电子转移和表面络合的复合物)降解机制,总结了活化过氧乙酸技术在废水、土壤或沉积物、地下水等环境介质中对污染物的降解效果。提出了未来研究重点,即开发高效稳定的活化过氧乙酸催化剂,加强活化过氧乙酸技术在土壤和沉积物中降解有机污染物机制的探索,深入联合其他处理技术的应用研究。
Abstract:The frequent detection of emerging contaminants poses a potential risk to ecosystems and human health, such as antibiotics and endocrine disruptors in the environment in recent years. The research and development of efficient and stable organic pollutant control technology is a research hotspot in the current environmental field. Taking the advanced oxidation technology of activated peracetic acid (PAA) as the research example, the effectiveness of activated PAA through transition metals, carbon materials and their composites, and their degradation mechanism of organics were discussed, with emphasis on the degradation mechanism of free radicals (organic radicals, hydroxyl radicals) and non-radicals (singlet oxygen, high-valent metal-Oxo species, electron transfer and surface complexes). In addition, the application effect of activated PAA in wastewater, soil or sediments, groundwater, and other environmental media for the degradation of organic pollutants was summarized. Finally, future research focuses were proposed, including developing the catalysts of activated PAA with efficiency and stability, strengthening the mechanism exploration of activated PAA to degrade organic pollutants in soil and sediment, and deepening the application studies of combined treatment technologies.
-
Key words:
- peracetic acid (PAA) /
- transition metals /
- carbon materials /
- composite materials /
- degrading mechanism
-
表 1 均相过渡金属活化PAA降解有机污染物的效能
Table 1. Effectiveness of homogeneous transition-metal activated PAA to degrade organic pollutants
活化PAA的
催化剂降解的有机污染物 反应条件 降解率/% 数据来源 名称 浓度/(µmol/L) Fe(Ⅱ) 亚甲基蓝、萘普生、双酚A 15 催化剂浓度为100 μmol/L,PAA浓度为 100 μmol/L,
pH为3.0,温度为22 ℃,时间为10 min89.4、98.2、87.7 文献[13] Co(Ⅱ) 卡马西平 15 催化剂浓度为10 μmol/L,PAA浓度为100 μmol/L,
pH为7.1,温度为22 ℃,时间为30 min97.7 文献[15] Fe(Ⅱ) 双氯芬酸 1 催化剂浓度为5 mg/L,PAA浓度为100 μmol/L,
pH为7.0,温度为25 ℃,时间为1 min80 文献[16] Fe(Ⅵ) 卡马西平 10 催化剂浓度为200 μmol/L,PAA浓度为100 μmol/L,
pH为9.0,温度为(25±1)℃,时间为1 min100 文献[17] Cu2+强化UV 双氯芬酸 1 催化剂浓度为5 μmol/L,PAA浓度为50 μmol/L,
pH为7.0,时间为20 min96 文献[18] Cu(Ⅱ)协同热 双氯芬酸 5 催化剂浓度为10 μmol/L,PAA浓度为0.55 mmol/L,
pH为8,温度为60 °C,时间为13 min100 文献[19] FeCl3 罗丹明B 501) 催化剂浓度为50 mg/L,PAA浓度为50 mg/L,
pH为3.0,时间为10 min99.9 文献[20] 1)单位为mg/L。 表 2 非均相过渡金属活化PAA降解有机污染物的效能
Table 2. Effectiveness of heterogeneous transition-metal activated PAA to degrade organic pollutants
活化PAA的催化剂 降解的有机污染物 反应条件 降解率/% 数据
来源名称 浓度/(µmol/L) S-Fe0 磺胺二甲嘧啶 51) 催化剂浓度为20 mg/L,PAA浓度为100 µmol/L,pH为4.0,时间为60 min 86.5 文献[21] FeS SMX 10 催化剂浓度为25 mg/L,PAA浓度为100 µmol/L,pH为7.0,时间为10 min 93.08 文献[22] 零价铜 双氯芬酸 1 催化剂浓度为0.5 g/L,PAA浓度为100 µmol/L,pH为3.0,
温度为25 ℃,时间为40 min95.5 文献[23] 纳米CuO 卡马西平 4.23 催化剂浓度为40 mg/L,PAA浓度为0.52 mmol/L,pH为7.0,时间为30 min 87 文献[24] CuFeS2 甲硝唑 101) 催化剂浓度为4 g/L,PAA浓度为460 µmol/L,pH为3.0,时间为30 min 83.92 文献[25] Co3O4 橙G 50 催化剂浓度为 0.1 g/L,PAA浓度为0.5 mmol/L,pH为7.0,时间为90 min 100 文献[26] FeCo2O4 SMX 10 催化剂浓度为0.1 g/L,PAA浓度为100 µmol/L,pH为7.0,
温度为25 ℃,时间为60 min97 文献[28] Co1.1Mn1.9O4 SMX 10 催化剂浓度为25 mg/L,PAA浓度为0.26 mmol/L,pH为7.0,
温度为20 ℃,时间为7 min100 文献[29] CoFe2O4 罗丹明B 20 1) 催化剂浓度为 0.5 g/L,PAA浓度为0.8 mmol/L,pH为7.0,时间为10 min 95 文献[30] 零价钴 SMX 5 催化剂浓度为0.1 g/L,PAA浓度为50 µmol/L,pH为7.0,时间为10 min 99.4 文献[31] 零价钴 罗丹明B 20 1) 催化剂浓度为0.1 g/L,PAA浓度为0.8 mmol/L,pH为7.0,时间为3 min 98.3 文献[32] LaCoO3 SMX 50 催化剂浓度为20 mg/L,PAA浓度为0.66 mmol/L,pH为7.0,
温度为25 ℃,时间为60 min100 文献[33] 1)单位为mg/L。 表 3 碳材料活化PAA降解有机污染物的效果
Table 3. Effectiveness of carbon material activated PAA to degrade organic pollutants
活化PAA的催化剂 降解的有机污染物 反应条件 降解率/% 数据来源 名称 浓度/(μmol/L) 活性炭纤维 活性艳红
X-3B50 催化剂浓度为2 g/L,PAA浓度为5 mmol/L,
pH=7.0,温度为25 ℃,时间为45 min97 文献[4] 氮掺杂还原石墨烯 SMX 150 催化剂浓度为0.5 g/L,PAA浓度为1 mmol/L,
pH为3.0,温度为25 ℃,时间为60 min96 文献[34] 氮掺杂氧化石墨烯 苯酚 11) 催化剂浓度为30 mg/L,PAA浓度为25 mg/L,
pH为7.0,时间为60 min100 文献[35] 剩余污泥制备碳基材料 4-氯苯酚 51) 催化剂浓度为25 mg/L,PAA浓度为6 mmol/L,
pH为7.0,时间为90 min91.2 文献[36] 花生壳基生物炭 乙酰氨基酚 100 催化剂浓度为0.2 g/L,PAA浓度为4 mmol/L,
pH为5.0,时间为20 min92.8 文献[37] 碳化聚苯胺 苯酚 10 催化剂浓度为25 mg/L,PAA浓度为0.1 mmol/L,
pH为7.0,时间为60 min96 文献[38] 热改性活性炭 SMX 79 催化剂浓度为50 mg/L,PAA浓度为0.26 mmol/L,
pH为7.0,时间为90 min99.4 文献[39] 碳纳米管 偶氮染料 201) 催化剂浓度为0.1 g/L,PAA浓度为0.02 g/L,
pH为7.0,时间为180 min≥90 文献[40] 氧化还原石墨烯 SMX 10 催化剂浓度为0.1 g/L,PAA浓度为100 μmol/L,
pH为5,温度为(25±2)℃,时间为5 min95 文献[41] 1)单位为mg/L。 表 4 复合材料活化PAA降解有机污染物的效果
Table 4. Effectiveness of composite materials activated PAA to degrade organic pollutants
活化PAA的催化剂 降解的有机污染物 反应条件 降解率/% 数据来源 名称 浓度/(μmol/L) Fe负载生物质炭 酸性橙色染料 143 催化剂浓度为0.3 g/L,PAA浓度为1.144 mol/L,
pH为7.0,时间为25 min93.3 文献[42] Fe2O3改性蒙脱石 2,4-二氯苯酚 1001) 催化剂浓度为1 g/L,PAA浓度为0.02 mol/L,
pH为7.0,温度为25 ℃,时间为210 min70 文献[43] CoFe2S4-CN 罗丹明B 40 催化剂浓度为20 mg/L,PAA浓度为1 mmol/L,
pH为6.5,温度为25 ℃,时间为60 min99.1 文献[44] Co@微米零价铁 SMX 20 催化剂浓度为0.1 g/L,PAA浓度为200 µmol/L,
pH为7.0,时间为30 min96 文献[45] RuO2/MWCNTs SMX 50 催化剂浓度为0.2 g/L,PAA浓度为1 mmol/L,
pH为7,时间为15 min100 文献[46] 金属有机骨架(ZIF)-67 磺胺氯哒嗪 10 催化剂浓度为0.05 g/L,PAA浓度为50 µmol/L,
pH为7.0,温度为25 ℃,时间为3 min100 文献[47] CoFe2O4/CuO SMX 10 催化剂浓度为20 mg/L,PAA浓度为200 µmol/L,
pH为7.0,温度为31 ℃,时间为10 min92 文献[48] 氮化碳负载FeCo2S4 罗丹明B 40 PAA浓度为1 mmol/L,pH为6.5,温度为25 ℃,时间为60 min 100 文献[49] 混合Fe(Ⅱ)/Fe(Ⅲ)价态
MIL-53(Fe)对硝基苯酚 201) 催化剂浓度为20 mg/L,PAA浓度为5 mol/L,
pH为7.0,温度为20 ℃,时间为120 min100 文献[50] CoFe2O4@木质素
衍生生物质炭SMX 101) 催化剂浓度为0.1 g/L,PAA浓度为550 µmol/L,
pH 为7.0,温度为25 ℃,时间为60 min95.8 文献[51] 1)单位为mg/L。 表 5 活化过氧乙酸技术在降解废水有机污染物的应用
Table 5. Application of activated PAA technology to degrade organic pollutants in wastewater
降解的有机污染物 PAA浓度/
(mmol/L)活化剂 pH 活性物种 去除率/% 数据
来源类型 名称 浓度/(µmol/L) 种类 剂量 酚类
有机物苯酚 10 0.4 Co(Ⅱ) 0.01 mmol/L 7.0 CH3C(O)OO· 74.1(10 min) 文献[58] 对硝基苯酚 201) 5 000 混合Fe(Ⅱ)/Fe(Ⅲ)
价态MIL-53(Fe)20 mg/L 7 ·OH 100(120 min) 文献[50] 苯酚 10 0.1 碳化聚苯胺 25 mg/L 7 1O2 96(60 min) 文献[38] 染料 亚甲蓝 31.26 3.6 电化学(EC) 铂片和石墨板电极,电解液Na2NO3浓度为0.45 g/L;电流密度为10 mA/cm2 3 ·OH、CH3C(O)O·和 CH3C(O)OO· 93.99(120 min) 文献[59] 橙G 50 0.5 Co3O4 100 mg/L 7 CH3C(O)O·和 CH3C(O)OO· 100(90 min) 文献[26] 偶氮染料 201) 201) 碳纳米管 100 mg/L 7 未提及 >90(180 min) 文献[40] 药物 卡马西平 4.23 0.52 纳米CuO 40 mg/L 7.0 CH3C(O)OO· 87(30 min) 文献[24] 土霉素 ≤10.86 0.066 UV 波长为254 nm, 照射剂量为0~223.2 mJ/cm2 7.1 ·OH 100(45 min) 文献[60] 诺氟沙星 6.26 0.131 中压紫外线
(MPUV)波长为200~300 nm,照射剂量为0~500 mJ/cm2 9 ·OH、·O2-和1O2 96.60(50 min) 文献[61] 三氯生 1 1 UV-Fe2+ Fe2+浓度为0.56 mg/L,
波长为254 nm,
光强度为0.24 mW/cm23.5 ·OH、CH3C(O)O·和CH3C(O)OO· 100(20 min) 文献[62] 磺胺二甲嘧啶 35.93 0.1 UV/Fe0 Fe0浓度为0.1 g/L,
波长为254 nm,
紫外灯功率为6 W4 ·OH、CH3C(O)O·和CH3C(O)OO· 85(60 min) 文献[63] SMX 5 0.2 热 60 ℃ 7 CH3C(O)O·和CH3C(O)OO· 86(25 min) 文献[9] SMX 5 400 Fe2+-沸石 800 mg/L 7 ·OH 100(50 min) 文献[11] SMX 50 0.66 LaCoO3 20 mg/L 7 CH3C(O)O·和 CH3C(O)OO· 100(60 min) 文献[33] SMX 10 0.55 CoFe2O4@生物质炭 100 mg/L 7.0±0.2 CH3C(O)O·和CH3C(O)OO· 95.8(60 min) 文献[51] 注:本表所列均为降解反应的最佳条件。1)单位为mg/L。 表 6 活化过氧乙酸技术在降解土壤和沉积物中有机污染物的应用
Table 6. Application of activated PAA technology to degrade organic pollutants in soil and sediment
降解的有机污染物 氧化剂 土壤/沉积物基本情况 pH 影响去除
效果的因素去除效果 类型 名称 浓度 湖泊沉
积物[64]α-甲基萘 10~25 mmol/kg 去离子水、乙酸、H2O2溶液体积比为1:1:1的混合物 总有机碳含量为2.1%~
12.8%,表面积为3.2~
22.0 m2/g7.49~7.67 沉积物的表面积和有机碳含量 24 h,沙质沉积物和粉质黏土沉积物中α-甲基萘的去除率分别为70%和100% 湖泊沉
积物[65]苯并[a]芘 10~25 mmol/kg H2O2、乙酸、去离子水体积比为1:1:1的混合物 沉积物Ⅰ,大部分颗粒粒径<75 μm,有机碳含量为12.6%,表面积为14.0 m2/g;沉积物Ⅱ,沙质类型,大部分颗粒粒径为75~850 μm,有机碳含量为0.5%,表面积为1.2 m2/g 约7 沉积物的粒径、表面积和有机碳含量 24 h,苯并[a]芘的去除率均为100%,其中,沉积物Ⅰ中的反应速率为沉积物Ⅱ的1.5倍 超级基金污染场地(Superfund)[66] 多环芳烃(PAHs) 密歇根湖西南海岸的污染土壤(Bedford LT)和Bedford LT10号场地的PAH浓度分别为500~
1 000、2 000~
3 000 mg/kgH2O2、乙酸、去离子水体积比为3:5:7或3:3:9的混合物 Bedford LT 10和Bedford LT土壤的含水量分别为25%和18.5%,TOC浓度分别为11%和18.5%,pH分别为7.09和7.04 约7 沉积物的表面积和有机碳含量 24 h,Bedford LT
的14种PAHs几乎完全降解;
Bedford LT10号未观察到14种PAHs的降解沙质和粉质黏土沉
积物[67]α-甲基萘和
苯并[a]芘α-甲基萘或苯并[a]芘浓度为500 mg/kg H2O2、乙酸、去离子水体积比为2:5:8的混合物 沙质沉积物,颗粒粒径>150 μm;粉质黏土,颗粒粒径为75~150 μm,总有机碳浓度分别为0.5%和1.4% 约7 沉积物粒径和有机碳含量 24 h,α-甲基萘的去除率为90%;苯并[a]芘的去除率为90% -
[1] 敖蒙蒙, 魏健, 陈忠林, 等.四环素类抗生素环境行为及其生态毒性研究进展[J]. 环境工程技术学报,2021,11(2):314-324. doi: 10.12153/j.issn.1674-991X.20200096AO M M, WEI J, CHEN Z L, et al. Research progress on environmental behaviors and ecotoxicity of tetracycline antibiotics[J]. Journal of Environmental Engineering Technology,2021,11(2):314-324. doi: 10.12153/j.issn.1674-991X.20200096 [2] ZHANG K J, ZHOU X Y, DU P H, et al. Oxidation of β-lactam antibiotics by peracetic acid: reaction kinetics, product and pathway evaluation[J]. Water Research,2017,123:153-161. doi: 10.1016/j.watres.2017.06.057 [3] 王静晓, 朱柯安, 陈飞.氯离子活化过氧乙酸对罗丹明B的降解性能及机理研究[J]. 环境科学研究,2021,34(12):2850-2858. doi: 10.13198/j.issn.1001-6929.2021.09.19WANG J X, ZHU K A, CHEN F. Degradation performance and mechanism of rhodamine B by chloride activated peracetic acid[J]. Research of Environmental Sciences,2021,34(12):2850-2858. doi: 10.13198/j.issn.1001-6929.2021.09.19 [4] ZHOU F Y, LU C, YAO Y Y, et al. Activated carbon fibers as an effective metal-free catalyst for peracetic acid activation: implications for the removal of organic pollutants[J]. Chemical Engineering Journal,2015,281:953-960. doi: 10.1016/j.cej.2015.07.034 [5] HOLLMAN J, DOMINIC J A, ACHARI G. Degradation of pharmaceutical mixtures in aqueous solutions using UV/peracetic acid process: Kinetics, degradation pathways and comparison with UV/H2O2[J]. Chemosphere,2020,248:125911. doi: 10.1016/j.chemosphere.2020.125911 [6] RIZZO L, LOFRANO G, GAGO C, et al. Antibiotic contaminated water treated by photo driven advanced oxidation processes: ultraviolet/H2O2 vs ultraviolet/peracetic acid[J]. Journal of Cleaner Production,2018,205:67-75. doi: 10.1016/j.jclepro.2018.09.101 [7] RIZZO L, AGOVINO T, NAHIM-GRANADOS S, et al. Tertiary treatment of urban wastewater by solar and UV-C driven advanced oxidation with peracetic acid: effect on contaminants of emerging concern and antibiotic resistance[J]. Water Research,2019,149:272-281. doi: 10.1016/j.watres.2018.11.031 [8] KUNIGK L, GOMES D R, FORTE F, et al. The influence of temperature on the decomposition kinetics of peracetic acid in solutions[J]. Brazilian Journal of Chemical Engineering,2001,18(2):217-220. doi: 10.1590/S0104-66322001000200009 [9] WANG J W, WAN Y, DING J Q, et al. Thermal activation of peracetic acid in aquatic solution: the mechanism and application to degrade sulfamethoxazole[J]. Environmental Science & Technology,2020,54(22):14635-14645. [10] DENG J W, LIU S L, FU Y S, et al. Heat-activated peracetic acid for degradation of diclofenac: kinetics, influencing factors and mechanism[J]. Environmental Technology,2023,44(19):2946-2954. doi: 10.1080/09593330.2022.2048086 [11] WANG S X, WANG H B, LIU Y Q, et al. Effective degradation of sulfamethoxazole with Fe2+-zeolite/peracetic acid[J]. Separation and Purification Technology,2020,233:115973. doi: 10.1016/j.seppur.2019.115973 [12] LUUKKONEN T, PEHKONEN S O. Peracids in water treatment: a critical review[J]. Critical Reviews in Environmental Science and Technology,2017,47(1):1-39. doi: 10.1080/10643389.2016.1272343 [13] KIM J, ZHANG T Q, LIU W, et al. Advanced oxidation process with peracetic acid and Fe(Ⅱ) for contaminant degradation[J]. Environmental Science & Technology,2019,53(22):13312-13322. [14] 田丹, 吴玮, 沈芷璇, 等.Co(Ⅱ)活化过氧乙酸降解有机染料研究[J]. 环境科学学报,2018,38(10):4023-4031. doi: 10.13671/j.hjkxxb.2018.0170TIAN D, WU W, SHEN Z X, et al. Degradation of organic dyes with peracetic acid activated by Co(Ⅱ)[J]. Acta Scientiae Circumstantiae,2018,38(10):4023-4031. doi: 10.13671/j.hjkxxb.2018.0170 [15] KIM J, DU P H, LIU W, et al. Cobalt/peracetic acid: advanced oxidation of aromatic organic compounds by acetylperoxyl radicals[J]. Environmental Science & Technology,2020,54(8):5268-5278. [16] WANG Z R, SHI H L, WANG S X, et al. Degradation of diclofenac by Fe(Ⅱ)-activated peracetic acid[J]. Environmental Technology,2021,42(27):4333-4341. doi: 10.1080/09593330.2020.1756926 [17] MANOLI K, LI R B, KIM J, et al. Ferrate(Ⅵ)-peracetic acid oxidation process: rapid degradation of pharmaceuticals in water[J]. Chemical Engineering Journal,2022,429:132384. doi: 10.1016/j.cej.2021.132384 [18] 张李. 基于过氧乙酸和过一硫酸盐的高级氧化技术去除水中双氯芬酸的研究[D]. 成都: 西南交通大学, 2021. [19] 邓杰文, 张琳悦, 付永胜, 等. Cu(Ⅱ)协同热活化过氧乙酸降解水中双氯芬酸[J/OL]. 环境化学: 1-8[2023-03-16]. https://kns.cnki.net/kcms/detail/11.1844.X.20221103.1031.002.html. [20] RAHMANI A R, GILAN R A, ASGARI G, et al. Enhanced degradation of Rhodamine B dye by Fenton/peracetic acid and photo-Fenton/peracetic acid processes[J]. International Journal of Chemical Reactor Engineering,2022,20(12):1251-1260. doi: 10.1515/ijcre-2022-0008 [21] PAN Y W, BU Z Y, LI J, et al. Sulfamethazine removal by peracetic acid activation with sulfide-modified zero-valent iron: efficiency, the role of sulfur species, and mechanisms[J]. Separation and Purification Technology,2021,277:119402. doi: 10.1016/j.seppur.2021.119402 [22] YANG S R, HE C S, XIE Z H, et al. Efficient activation of PAA by FeS for fast removal of pharmaceuticals: the dual role of sulfur species in regulating the reactive oxidized species[J]. Water Research,2022,217:118402. doi: 10.1016/j.watres.2022.118402 [23] ZHANG L, FU Y S, WANG Z R, et al. Removal of diclofenac in water using peracetic acid activated by zero valent copper[J]. Separation and Purification Technology,2021,276:119319. doi: 10.1016/j.seppur.2021.119319 [24] ZHANG L L, CHEN J B, ZHANG Y L, et al. Highly efficient activation of peracetic acid by nano-CuO for carbamazepine degradation in wastewater: the significant role of H2O2 and evidence of acetylperoxy radical contribution[J]. Water Research,2022,216:118322. doi: 10.1016/j.watres.2022.118322 [25] YANG K, ZHAI Z H, LIU H L, et al. Peracetic acid activation by natural chalcopyrite for metronidazole degradation: unveiling the effects of Cu-Fe bimetallic sites and sulfur species[J]. Separation and Purification Technology,2023,305:122500. doi: 10.1016/j.seppur.2022.122500 [26] WU W, TIAN D, LIU T C, et al. Degradation of organic compounds by peracetic acid activated with Co3O4: a novel advanced oxidation process and organic radical contribution[J]. Chemical Engineering Journal,2020,394:124938. doi: 10.1016/j.cej.2020.124938 [27] HU P D, LONG M C. Cobalt-catalyzed sulfate radical-based advanced oxidation: a review on heterogeneous catalysts and applications[J]. Applied Catalysis B:Environmental,2016,181:103-117. doi: 10.1016/j.apcatb.2015.07.024 [28] MENG L, DONG J Y, CHEN J, et al. Activation of peracetic acid by spinel FeCo2O4 nanoparticles for the degradation of sulfamethoxazole[J]. Chemical Engineering Journal,2023,456:141084. doi: 10.1016/j.cej.2022.141084 [29] ZHANG L L, CHEN J B, ZHENG T L, et al. Co-Mn spinel oxides trigger peracetic acid activation for ultrafast degradation of sulfonamide antibiotics: Unveiling critical role of Mn species in boosting Co activity[J]. Water Research,2023,229:119462. doi: 10.1016/j.watres.2022.119462 [30] ZHOU G F, FU Y S, ZHOU R Y, et al. Efficient degradation of organic contaminants by magnetic cobalt ferrite combined with peracetic acid[J]. Process Safety and Environmental Protection,2022,160:376-384. doi: 10.1016/j.psep.2022.02.031 [31] ZHOU G F, ZHOU R Y, LIU Y Q, et al. Efficient degradation of sulfamethoxazole using peracetic acid activated by zero-valent cobalt[J]. Journal of Environmental Chemical Engineering,2022,10(3):107783. doi: 10.1016/j.jece.2022.107783 [32] 周高峰, 周润宇, 刘义青, 等.零价钴活化过氧乙酸降解水中罗丹明B的研究[J]. 环境科学学报,2022,42(11):47-55.ZHOU G F, ZHOU R Y, LIU Y Q, et al. Degrdation of rhodamine B by peracetic acid activated with zero-valent cobalt[J]. Acta Scientiae Circumstantiae,2022,42(11):47-55. [33] ZHOU X F, WU H W, ZHANG L L, et al. Activation of peracetic acid with lanthanum cobaltite perovskite for sulfamethoxazole degradation under a neutral pH: the contribution of organic radicals[J]. Molecules,2020,25(12):2725. doi: 10.3390/molecules25122725 [34] 卢建. 基于过氧化物的高级氧化体系降解水体中抗生素的研究[D]. 苏州: 苏州科技大学, 2019. [35] 刘永泽, 田幸, 张立秋, 等. 一种采用氮掺杂碳材料活化过氧乙酸降解水中有机污染物的方法: CN111606405A[P]. 2020-09-01. [36] 马军, 程平统, 吴丽颖, 孙志强, 甄宇菲, 李璐玮, 佘月城. 一种以剩余污泥制备碳基催化剂的制备方法及应用: CN113209970A[P]. 2023-05-05. [37] 张晖, 樊晓辉, 吴飞, 苗菲, 赵津津. 一种利用不同炭材料活化过氧乙酸经电子转移机制去除水中污染物的方法及其应用: CN113860472A[P]. 2022-11-01. [38] TIAN X, LIU S Q, ZHANG B N, et al. Carbonized polyaniline-activated peracetic acid advanced oxidation process for organic removal: efficiency and mechanisms[J]. Environmental Research,2023,219:115035. doi: 10.1016/j.envres.2022.115035 [39] DAI C M, LI S, DUAN Y P, et al. Mechanisms and product toxicity of activated carbon/peracetic acid for degradation of sulfamethoxazole: implications for groundwater remediation[J]. Water Research,2022,216:118347. doi: 10.1016/j.watres.2022.118347 [40] 陈家斌, 黄天寅, 沈芷璇, 等. 一种碳纳米管活化过氧乙酸降解废水中污染物的方法: CN108423793A[P]. 2018-08-21. [41] KONG D Z, ZHAO Y M, FAN X R, et al. Reduced graphene oxide triggers peracetic acid activation for robust removal of micropollutants: the role of electron transfer[J]. Environmental Science & Technology,2022,56(16):11707-11717. [42] SHI C J, WANG Y, ZHANG K, et al. Fe-biochar as a safe and efficient catalyst to activate peracetic acid for the removal of the acid orange dye from water[J]. Chemosphere,2022,307:135686. doi: 10.1016/j.chemosphere.2022.135686 [43] VIRKUTYTE J, VARMA R S. Eco-friendly magnetic iron oxide-pillared montmorillonite for advanced catalytic degradation of dichlorophenol[J]. ACS Sustainable Chemistry & Engineering,2014,2(7):1545-1550. [44] ZHOU R Y, FU Y S, ZHOU G F, et al. Heterogeneous degradation of organic contaminants by peracetic acid activated with FeCo2S4 modified g-C3N4: identification of reactive species and catalytic mechanism[J]. Separation and Purification Technology,2022,282:120082. doi: 10.1016/j.seppur.2021.120082 [45] YANG L W, SHE L H, XIE Z H, et al. Boosting activation of peracetic acid by Co@mZVI for efficient degradation of sulfamethoxazole: interesting two-phase generation of reactive oxidized species[J]. Chemical Engineering Journal,2022,448:137667. doi: 10.1016/j.cej.2022.137667 [46] QIAN Y J, HUANG J J, CHEN J B, et al. Activation of peracetic acid by RuO2/MWCNTs to degrade sulfamethoxazole at neutral condition[J]. Chemical Engineering Journal,2022,431:134217. doi: 10.1016/j.cej.2021.134217 [47] DUAN J, CHEN L, JI H D, et al. Activation of peracetic acid by metal-organic frameworks (ZIF-67) for efficient degradation of sulfachloropyridazine[J]. Chinese Chemical Letters,2022,33(6):3172-3176. doi: 10.1016/j.cclet.2021.11.072 [48] 刘振中, 万思文, 吴阳, 等. CoFe2O4/CuO活化过氧乙酸高效降解磺胺甲恶唑[J/OL]. 物理化学学报: 1-10[2023-03-16]. https://kns.cnki.net/kcms/detail/11.1892.O6.20221226.1320.001.html. [49] 周润宇, 付永胜, 周高峰, 等.石墨相氮化碳负载尖晶石型铁钴硫化物活化过氧乙酸降解自然水体中罗丹明B的研究[J]. 水处理技术,2022,48(12):77-82. doi: 10.16796/j.cnki.1000-3770.2022.12.015ZHOU R Y, FU Y S, ZHOU G F, et al. Degradation of rhodamine B in natural waters by peracetic acid activated with FeCo2S4 modified g-C3N4[J]. Technology of Water Treatment,2022,48(12):77-82. doi: 10.16796/j.cnki.1000-3770.2022.12.015 [50] 胡倩, 杨涛语, 朱斐超, 等.混合价态铁基金属有机框架催化过氧乙酸高效降解对硝基苯酚[J]. 纺织学报,2022,43(11):133-140.HU Q, YANG T Y, ZHU F C, et al. Peracetic acid activation for efficient degradation of p-nitrophenol by mixed-valence iron-based metal-organic framework[J]. Journal of Textile Research,2022,43(11):133-140. [51] DONG J, XU W H, LIU S B, et al. Lignin-derived biochar to support CoFe2O4: effective activation of peracetic acid for sulfamethoxazole degradation[J]. Chemical Engineering Journal,2022,430:132868. doi: 10.1016/j.cej.2021.132868 [52] CHEN J C, PAVLOSTATHIS S G. Peracetic acid fate and decomposition in poultry processing wastewater streams[J]. Bioresource Technology Reports,2019,7:100285. doi: 10.1016/j.biteb.2019.100285 [53] AO X W, ELORANTA J, HUANG C H, et al. Peracetic acid-based advanced oxidation processes for decontamination and disinfection of water: a review[J]. Water Research,2021,188:116479. doi: 10.1016/j.watres.2020.116479 [54] ZHANG T Q, HUANG C H. Modeling the kinetics of UV/peracetic acid advanced oxidation process[J]. Environmental Science & Technology,2020,54(12):7579-7590. [55] CARETTI C, LUBELLO C. Wastewater disinfection with PAA and UV combined treatment: a pilot plant study[J]. Water Research,2003,37(10):2365-2371. doi: 10.1016/S0043-1354(03)00025-3 [56] DAI Y H, QI C D, CAO H, et al. Enhanced degradation of sulfamethoxazole by microwave-activated peracetic acid under alkaline condition: influencing factors and mechanism[J]. Separation and Purification Technology,2022,288:120716. doi: 10.1016/j.seppur.2022.120716 [57] LIU B H, GUO W Q, JIA W R, et al. Novel nonradical oxidation of sulfonamide antibiotics with co(Ⅱ)-doped g-C3N4-activated peracetic acid: role of high-valent cobalt–oxo species[J]. Environmental Science & Technology,2021,55(18):12640-12651. [58] DU P H, WANG J J, SUN G D, et al. Hydrogen atom abstraction mechanism for organic compound oxidation by acetylperoxyl radical in Co(Ⅱ)/peracetic acid activation system[J]. Water Research,2022,212:118113. doi: 10.1016/j.watres.2022.118113 [59] YUAN D L, YANG K, PAN S Y, et al. Peracetic acid enhanced electrochemical advanced oxidation for organic pollutant elimination[J]. Separation and Purification Technology,2021,276:119317. doi: 10.1016/j.seppur.2021.119317 [60] YAN T T, PING Q, ZHANG A, et al. Enhanced removal of oxytetracycline by UV-driven advanced oxidation with peracetic acid: insight into the degradation intermediates and N-nitrosodimethylamine formation potential[J]. Chemosphere,2021,274:129726. doi: 10.1016/j.chemosphere.2021.129726 [61] AO X W, WANG W B, SUN W J, et al. Degradation and transformation of norfloxacin in medium-pressure ultraviolet/peracetic acid process: an investigation of the role of pH[J]. Water Research,2021,203:117458. doi: 10.1016/j.watres.2021.117458 [62] WANG S X, CHEN Z, WANG Z R, et al. Enhanced degradation of triclosan using UV-Fe2+synergistic activation of peracetic acid[J]. Environmental Science:Water Research & Technology,2021,7(3):630-637. [63] WANG L Q, YE J Y, ZHANG J Y, et al. Removal of sulfamethazine using peracetic acid activated by Fe0 and UV: efficiency and mechanism study[J]. Journal of Environmental Chemical Engineering,2021,9(6):106358. doi: 10.1016/j.jece.2021.106358 [64] LEVITT J S, N’GUESSAN A L, RAPP K L, et al. Remediation of α-methylnaphthalene-contaminated sediments using peroxy acid[J]. Water Research,2003,37(12):3016-3022. doi: 10.1016/S0043-1354(03)00116-7 [65] N'GUESSAN A L, LEVITT J S, NYMAN M C. Remediation of benzo(a)pyrene in contaminated sediments using peroxy-acid[J]. Chemosphere,2004,55(10):1413-1420. doi: 10.1016/j.chemosphere.2003.11.026 [66] SCOTT ALDERMAN N, N’GUESSAN A L, NYMAN M C. Effective treatment of PAH contaminated Superfund site soil with the peroxy-acid process[J]. Journal of Hazardous Materials,2007,146(3):652-660. doi: 10.1016/j.jhazmat.2007.04.068 [67] N'GUESSAN A L, CARIGNAN T, NYMAN M C. Optimization of the peroxy acid treatment of α-methylnaphthalene and benzo[a]pyrene in sandy and silty-clay sediments[J]. Environmental Science & Technology,2004,38(5):1554-1560. [68] LIN J B, HU Y Y, XIAO J Y, et al. Enhanced diclofenac elimination in Fe(Ⅱ)/peracetic acid process by promoting Fe(Ⅲ)/Fe(Ⅱ) cycle with ABTS as electron shuttle[J]. Chemical Engineering Journal,2021,420:129692. ⊗ doi: 10.1016/j.cej.2021.129692 -





 下载:
下载: