Preparation of modified sodium alginate gel material and its chromium removal performance
-
摘要:
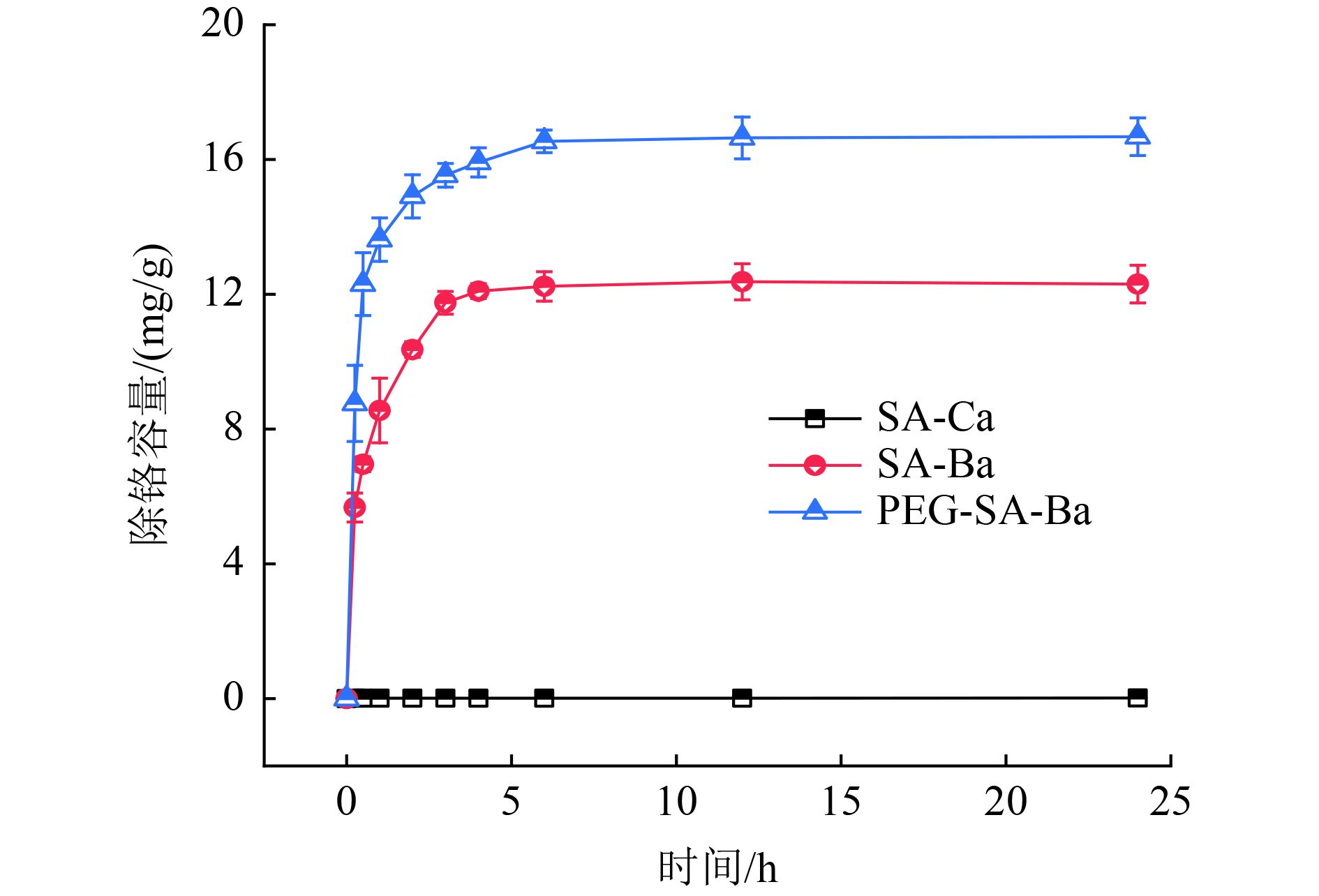
针对海藻酸钠凝胶(SA)材料在处理含铬废水时存在处理效果不理想、利用率低等问题,以海藻酸钠为原料,采用凝胶包埋法负载Ba2+制备凝胶球SA-Ba,并添加聚乙二醇进行共价混和改性,得到聚乙二醇改性海藻酸钡凝胶材料(PEG-SA-Ba)。利用所制材料进行除铬试验,与传统的海藻酸钙(SA-Ca)凝胶球对比,考察3种凝胶球材料除铬效果以及pH、初始Cr(Ⅵ)浓度、PEG-SA-Ba投加量等参数对PEG-SA-Ba材料除铬效果的影响,并通过扫描电子显微镜(SEM)、傅里叶红外光谱(FTIR)对样品进行表征。结果表明:海藻酸钠与Ba2+交联后得到的SA-Ba大大提高了SA的除铬效果,其除铬量是SA-Ca的800倍以上,经过共价改性后的PEG-SA-Ba为Cr(Ⅵ)的吸附提供了更多活性位点,除铬容量较未改性时提高了4.38 mg/g。随着pH的增加,PEG-SA-Ba对Cr(Ⅵ)的去除率也不断增大,pH为6时的除铬容量较pH为2时提高了16.24 mg/g,当pH超过8时,凝胶球结构不稳定易造成离子泄漏,导致在凝胶球之外有沉淀物生成。PEG-SA-Ba除铬容量随着Cr(Ⅵ)浓度增加而增加,随投加量增加而下降。SEM和FTIR表征分析验证了Ba2+负载成功且聚乙二醇与海藻酸钠交联效果良好。PEG-SA-Ba凝胶球材料在制备成本和方式上均具有明显优势,材料成本分别比SA和SA-Ba节约了99%和24%。
Abstract:Sodium alginate gel (SA) material has problems such as unsatisfactory treatment effect and low utilization rate in the treatment of chromium-containing wastewater. In order to solve these problems, sodium alginate was used as raw material, and gel balls (SA-Ba) were prepared by gel embedding method loaded with Ba2+, and polyethylene glycol was added for covalent mixing and modification to obtain polyethylene glycol modified barium alginate gel material (PEG-SA-Ba). Compared with the traditional calcium alginate (SA-Ca) gel ball, the chromium removal effect of the three gel ball materials and the effects of experimental parameters such as pH, initial Cr(Ⅵ) concentration, and PEG-SA-Ba dosage on the chromium removal effect of PEG-SA-Ba materials were investigated, and the samples were characterized by scanning electron microscopy (SEM) and Fourier infrared spectroscopy (FTIR). The results showed that: SA-Ba crosslinked with sodium alginate and Ba2+ greatly improved the chromium removal effect of SA, and the chromium removal amount was more than 800 times that of SA-Ca; the covalently modified PEG-SA-Ba provided more active sites for the adsorption of Cr(Ⅵ), and the chromium removal capacity was increased by 4.38 mg/g compared with that without modification. With the increase of pH, the removal rate of Cr(Ⅵ) by PEG-SA-Ba also continued to increase; the chromium removal capacity at pH 6 was 16.24 mg/g higher than that at pH 2, and when pH exceeds 8, the structure of the gel ball was unstable and easy to cause ion leakage, resulting in precipitate formation outside the gel ball. The chromium removal capacity of PEG-SA-Ba increased with the increase of Cr(Ⅵ) concentration and decreased with the increase of dosage. SEM and FTIR characterization verified that the Ba2+ loading was successful and the crosslinking effect of polyethylene glycol and sodium alginate was good. Finally, the discussion found that PEG-SA-BA gel ball materials had obvious advantages in preparation cost and method, saving more than 99% and 24% of material costs compared with SA and SA-Ba, respectively.
-
Key words:
- sodium alginate /
- gel balls /
- Cr(Ⅵ) /
- groundwater /
- adsorption
-
表 1 等温吸附拟合参数
Table 1. Isothermal adsorption fitting parameters
Langmuir模型 Freundlich模型 KL/(L/mg) qm/(mg/g) R2 KF/(L/mg) 1/n R2 0.0093 57.16 0.975 0.103 0.445 0.990 表 2 不同海藻酸钠基材料成本对比
Table 2. Cost comparison of different sodium alginate-based materials
-
[1] 王兴润, 李磊, 颜湘华, 等.铬污染场地修复技术进展[J]. 环境工程,2020,38(6):1-8. doi: 10.13205/j.hjgc.202006001WANG X R, LI L, YAN X H, et al. Progress in remediation of chromium-contaminated sites[J]. Environmental Engineering,2020,38(6):1-8. doi: 10.13205/j.hjgc.202006001 [2] WANG Z L, ZHANG H Z, WANG Y P, et al. Soil pollution investigation of chromium slag contaminated site[J]. Advanced Materials Research,2012,430/431/432:812-815. [3] SUH M, WIKOFF D, LIPWORTH L, et al. Hexavalent chromium and stomach cancer: a systematic review and meta-analysis[J]. Critical Reviews in Toxicology,2019,49(2):140-159. doi: 10.1080/10408444.2019.1578730 [4] de OLIVEIRA F L V, RUBIA K, PARRA D N F, et al. Potential oral exposure to low-dose chromium and stomach cancer mortality in the population in the interior of Sao Paulo State, Brazil[J]. Cadernos De Saude Publica,2021,37(4):1-10. [5] ALVAREZ C C, BRAVO GÓMEZ M E, HERNÁNDEZ ZAVALA A. Hexavalent chromium: regulation and health effects[J]. Journal of Trace Elements in Medicine and Biology,2021,65:126729. doi: 10.1016/j.jtemb.2021.126729 [6] 孟凡生, 王业耀, 汪春香, 等.铬污染地下水的PRB修复试验[J]. 工业用水与废水,2005,36(2):22-25. doi: 10.3969/j.issn.1009-2455.2005.02.007MENG F S, WANG Y Y, WANG C X, et al. A PRB remediation test of Cr-polluted groundwater[J]. Industrial Water & Wastewater,2005,36(2):22-25. doi: 10.3969/j.issn.1009-2455.2005.02.007 [7] BROWNLEE I A, ALLEN A, PEARSON J P, et al. Alginate as a source of dietary fiber[J]. Critical Reviews in Food Science and Nutrition,2005,45(6):497-510. doi: 10.1080/10408390500285673 [8] 张莉华, 李科, 卜方方.海藻酸钠在药物制剂中的应用进展[J]. 中南药学,2016,14(1):52-56. doi: 10.7539/j.issn.1672-2981.2016.01.013ZHANG L H, LI K, BU F F. Application of sodium alginate in pharmaceutic preparation[J]. Central South Pharmacy,2016,14(1):52-56. doi: 10.7539/j.issn.1672-2981.2016.01.013 [9] HUANG Y G, WANG Z Q. Preparation of composite aerogels based on sodium alginate, and its application in removal of Pb2+ and Cu2+ from water[J]. International Journal of Biological Macromolecules,2018,107:741-747. doi: 10.1016/j.ijbiomac.2017.09.057 [10] GAO X P, GUO C, HAO J J, et al. Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives[J]. International Journal of Biological Macromolecules,2020,164:4423-4434. doi: 10.1016/j.ijbiomac.2020.09.046 [11] WANG Z Q, HUANG Y G, WANG M, et al. Macroporous calcium alginate aerogel as sorbent for Pb2+ removal from water media[J]. Journal of Environmental Chemical Engineering,2016,4(3):3185-3192. doi: 10.1016/j.jece.2016.06.032 [12] 孙伟光, 邢佳, 邹海虹, 等.海藻酸钠凝胶球对铬(Ⅵ)的去除效果研究[J]. 现代农业科技,2015(10):208-209. doi: 10.3969/j.issn.1007-5739.2015.10.126SUN W G, XING J, ZOU H H, et al. Study on sodium alginate gel bead removing Cr(Ⅵ)[J]. Modern Agricultural Science and Technology,2015(10):208-209. doi: 10.3969/j.issn.1007-5739.2015.10.126 [13] 高娟娟, 王瑞杰, 万凯凯, 等.双金属离子交联剂对海藻酸盐凝胶性能的影响[J]. 化学工程,2021,49(7):1-5.GAO J J, WANG R J, WAN K K, et al. Effect of bimetallic ion crosslinkers on properties of alginate gel[J]. Chemical Engineering (China),2021,49(7):1-5. [14] UZAŞÇI S, TEZCAN F, ERIM B. Removal of hexavalent chromium from aqueous solution by barium ion cross-linked alginate beads[J]. International Journal of Enⅵronmental Science and Technology:(IJEST), 2014,11(7):1861-1868. [15] 胡嘉炜. 海藻酸钠基水凝胶的制备及其在污水处理中的应用研究[D]. 株洲: 湖南工业大学, 2020. [16] GIRIDHAR R S, PANDIT A S. Effect of curing agent on sodium alginate blends using barium chloride as crosslinking agent and study of swelling, thermal, and morphological properties[J]. International Journal of Polymeric Materials,2013,62(14):743-748. doi: 10.1080/00914037.2013.769236 [17] 朱文会, 王兴润, 董良飞, 等.海藻酸钠包覆型Fe-Cu双金属PRB填料的除Cr(Ⅵ)特性[J]. 化工学报,2013,64(9):3373-3380.ZHU W H, WANG X R, DONG L F, et al. Characteristics of removing Cr(Ⅵ) of Fe-Cu bimetal PRB coated by sodium alginate[J]. CIESC Journal,2013,64(9):3373-3380. [18] 陈维璞, 张恩浩, 林永波.海藻酸钠-钙-铁凝胶球对Cr2O72-吸附的研究[J]. 环境保护科学,2010,36(2):14-16. doi: 10.3969/j.issn.1004-6216.2010.02.005CHEN W P, ZHANG E H, LIN Y B. Study on sodium alginate-calcium-iron gel ball in Cr2O72- adsorption[J]. Environmental Protection Science,2010,36(2):14-16. doi: 10.3969/j.issn.1004-6216.2010.02.005 [19] 冯志云, 吴敏, 朱昌平, 等.聚乙二醇共价交联海藻酸钠凝胶制备及其药物缓释性能[J]. 材料科学与工程学报,2015,33(3):368-371.FENG Z Y, WU M, ZHU C P, et al. Preparation and sustained drug release of alginic acid gel covalently cross-linked by polyethylene glycol[J]. Journal of Materials Science and Engineering,2015,33(3):368-371. [20] DEWANGAN T, TIWARI A, BAJPAI A K. Removal of chromium(Ⅵ) ions by adsorption onto binary biopolymeric beads of sodium alginate and carboxymethyl cellulose[J]. Journal of Dispersion Science and Technology,2011,32(8):1075-1082. doi: 10.1080/01932691003659403 [21] BAJPAI J, SHRIVASTAVA R, BAJPAI A K. Dynamic and equilibrium studies on adsorption of Cr(Ⅵ) ions onto binary bio-polymeric beads of cross linked alginate and gelatin[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects,2004,236(1/2/3):81-90. [22] 高志敏. 海藻酸钠基复合吸附材料的制备及对重金属离子的吸附研究[D]. 济南: 山东建筑大学, 2016. [23] YUAN C B, ZHANG Y, YAO J S, et al. Facile synthesis of polyethylene Glycol@Tannin-amine microsphere towards Cr(Ⅵ) removal[J]. Polymers,2021,13(7):1035. doi: 10.3390/polym13071035 [24] MA W, WANG M Y, CHEN Z, et al. Characteristics of novel extraction and photoinduced precipitation approach by PEG/SA and fluorescence monitoring of Cr(Ⅵ)[J]. ACS Sustainable Chemistry & Engineering,2019,7(6):6323-6334. [25] 王旭, 杨欣楠, 黄币娟, 等.海藻酸钠负载硫化零价铁对水体中Cr(Ⅵ)的还原去除[J]. 环境科学,2021,42(6):2908-2916.WANG X, YANG X N, HUANG B J, et al. Sodium alginate loading of zero-valent iron sulfide for the reduction of Cr(Ⅵ) in water[J]. Environmental Science,2021,42(6):2908-2916. [26] 李华夏, 林毅, 周小斌, 等.生物炭负载纳米零价铁去除废水中重金属的研究进展[J]. 环境工程技术学报,2022,12(3):787-793. doi: 10.12153/j.issn.1674-991X.20210242LI H X, LIN Y, ZHOU X B, et al. Research progress on heavy metals removal from wastewater by biochar-supported nano zero-valent iron[J]. Journal of Environmental Engineering Technology,2022,12(3):787-793. doi: 10.12153/j.issn.1674-991X.20210242 [27] 朱文会, 董良飞, 王兴润, 等.Cr(Ⅵ)污染地下水修复的PRB填料实验研究[J]. 环境科学,2013,34(7):2711-2717. doi: 10.13227/j.hjkx.2013.07.044ZHU W H, DONG L F, WANG X R, et al. Experimental study on the remediation of chromium contaminated groundwater with PRB media[J]. Environmental Science,2013,34(7):2711-2717. doi: 10.13227/j.hjkx.2013.07.044 [28] 李思琪. Cr(Ⅵ)污染地下水修复的复合Fe~0-PRB填料实验研究[D]. 福州: 福州大学, 2018. [29] 朱文会, 王兴润, 董良飞, 等.海藻酸钠固定化Fe-Cu双金属去除Cr(Ⅵ)的作用机制[J]. 中国环境科学,2013,33(11):1965-1971.ZHU W H, WANG X R, DONG L F, et al. Mechanism of copper-iron bimetallic particles immobilized by sodium alginate in removal of Cr(Ⅵ)[J]. China Environmental Science,2013,33(11):1965-1971. [30] 潘虹, 王兴润, 王雷, 等.生物炭负载硫化改性纳米零价铁去除水中的Cr(Ⅵ)[J]. 环境工程技术学报,2023,13(2):663-668. doi: 10.12153/j.issn.1674-991X.20220250PAN H, WANG X R, WANG L, et al. Experimental study on the removal of Cr(Ⅵ) from water by biochar-based sulfide modification loaded with nano-zero valent iron[J]. Journal of Environmental Engineering Technology,2023,13(2):663-668. doi: 10.12153/j.issn.1674-991X.20220250 [31] 陈超, 刘建国, 赵光琪, 等.木质垃圾与废铁泥制备Fe0-BC材料及其对水中Cr(Ⅵ)的去除行为[J]. 环境科学研究,2023,36(5):995-1005.CHEN C, LIU J G, ZHAO G Q, et al. Preparation of Fe0-BC using wood waste and iron sludge and its application for removing Cr(Ⅵ)[J]. Research of Environmental Sciences,2023,36(5):995-1005. [32] JIANG D N, HUANG D L, LAI C, et al. Difunctional chitosan-stabilized Fe/Cu bimetallic nanoparticles for removal of hexavalent chromium wastewater[J]. Science of the Total Environment,2018,644:1181-1189. doi: 10.1016/j.scitotenv.2018.06.367 [33] QU M, CHEN H X, WANG Y, et al. Improved performance and applicability of copper-iron bimetal by sulfidation for Cr(Ⅵ) removal[J]. Chemosphere,2021,281:130820. doi: 10.1016/j.chemosphere.2021.130820 [34] GHEJU M. Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems[J]. Water, Air, & Soil Pollution,2011,222(1):103-148. [35] 马少云, 祝方, 商执峰.纳米零价铁铜双金属对铬污染土壤中Cr(Ⅵ)的还原动力学[J]. 环境科学,2016,37(5):1953-1959. doi: 10.13227/j.hjkx.2016.05.045MA S Y, ZHU F, SHANG Z F. Reduction kinetics of Cr(Ⅵ) in chromium contaminated soil by nanoscale zerovalent iron-copper bimetallic[J]. Environmental Science,2016,37(5):1953-1959. ⊗ doi: 10.13227/j.hjkx.2016.05.045 -





 下载:
下载: