The interactive effects of titanium dioxide nanoparticles and phosphate on arsenic accumulation and biotransformation in Chlamydomonas reinhardtii
-
摘要:
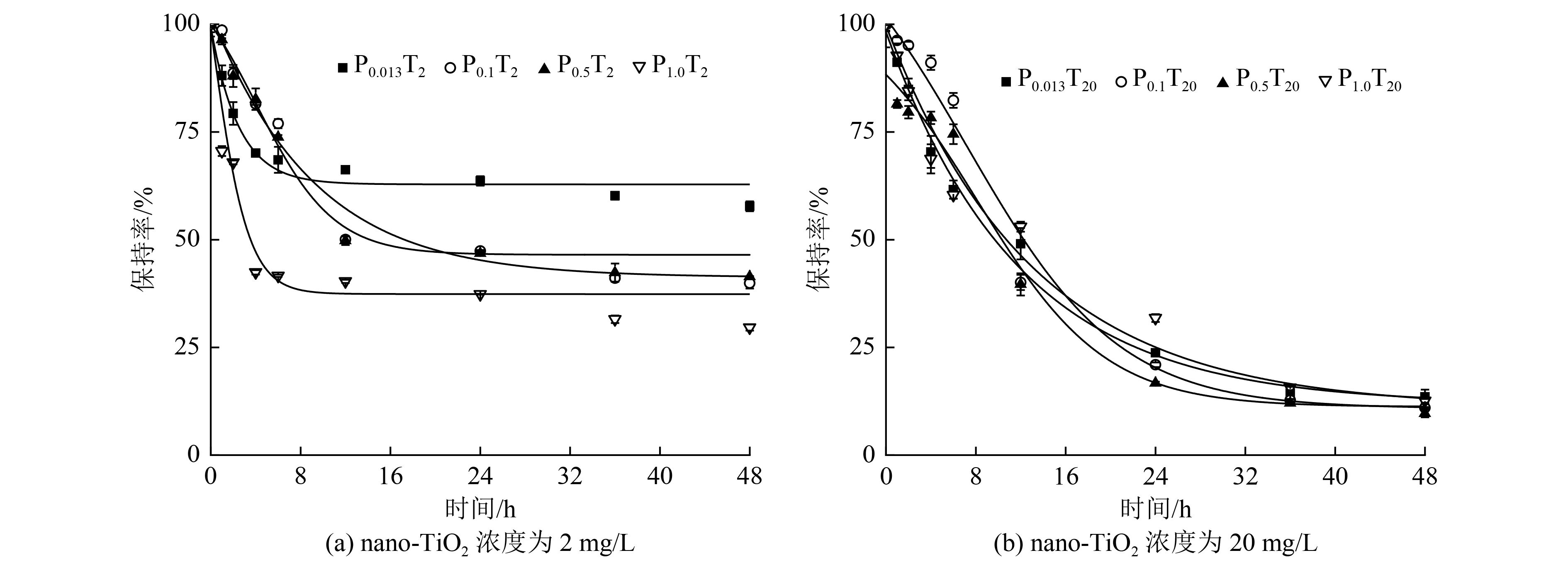
纳米材料因其较大的比表面积以及较强的反应活性,对砷(As)的环境行为具有一定的调控作用,而这可能对微藻As吸收代谢产生潜在的影响。以模式生物莱茵衣藻(Chlamydomonas reinhardtii)为研究对象,探究不同磷酸盐(PO4 3−)浓度下,纳米二氧化钛(nano-TiO2)对莱茵衣藻中As(Ⅴ)累积和生物转化的影响。结果表明:暴露初期(第1天)nano-TiO2作为载体显著促进了0.013、0.100和0.500 mmol/L PO4 3−处理组藻细胞对As的累积,但随着暴露时间的延长,nano-TiO2的载体效应呈下降趋势;暴露结束后(第8天),nano-TiO2添加组中,进入藻细胞的As(Ⅴ)除了还原成As(Ⅲ)及甲基化成二甲基砷外,还能进一步转化为一种可能为砷糖的未知化合物,且随着PO4 3−浓度的降低,藻细胞内这种砷糖所占比例逐渐增加,这可能会抑制As(Ⅲ)的外排;暴露结束后(第8天),培养基中主要检测到的As形态为As(Ⅴ)和As(Ⅲ),1.0和0.5 mmol/L处理组还有少量二甲基砷。nano-TiO2的添加降低了培养基中As(Ⅲ)的浓度,尤其是0.5和1.0 mmol/L PO4 3−处理组。研究结果表明,纳米材料与PO4 3−的互作可显著改变微藻As的累积与代谢过程。
Abstract:Nanomaterials can modify the environmental behavior of arsenic (As) due to their large specific surface area and high reaction activity, which may affect the absorption and metabolism of As in microalgae. In this study, Chlamydomonas reinhardtii was used as model organism to investigate the influence of titanium dioxide nanoparticles (nano-TiO2) on As(Ⅴ) accumulation and biotransformation in algal cells at different phosphate concentrations. The results showed that nano-TiO2 significantly promoted As accumulation in algae cells in 0.013, 0.100 and 0.500 mmol/L phosphate groups at the beginning of exposure (1 d), but the carrier effect of nano-TiO2 decreased with the extension of exposure time. After 8 days of exposure, in the nano-TiO2 addition groups, As(Ⅴ) in algal cells was not only reduced to As(Ⅲ) and methylated to dimethyl arsenic, but also further transformed to an unknown As compound, possibly arsenosugars. And the proportion of arsenosugars in algal cells gradually increased with the decrease of phosphate concentration, which might inhibit the efflux of As(Ⅲ). After 8 days of exposure, As(Ⅴ) and As(Ⅲ) were the main As species in the culture medium, and a small amount of dimethyl arsenic was also detected. The addition of nano-TiO2 decreased the proportions of As(Ⅲ) in the culture medium, especially in 0.5 and 1.0 mmol/L phosphate group. This study indicated that the interaction between nanomaterials and phosphate significantly affected the accumulation and metabolism of As in microalgae, which facilitates the application of microalgae in As remediation.
-
Key words:
- Chlamydomonas reinhardtii /
- arsenate /
- phosphate /
- nano-TiO2 /
- As species
-
表 1 试验分组设计
Table 1. Experimental group design
试验组别 As(Ⅴ)浓
度/(μmol/L)PO4 3−浓
度/(mmol/L)nano-TiO2浓
度/(mg/L)P0.013T0 10 0.013 0 P0.013T2 10 0.013 2 P0.013T20 10 0.013 20 P0.1T0 10 0.1 0 P0.1T2 10 0.1 2 P0.1T20 10 0.1 20 P0.5T0 10 0.5 0 P0.5T2 10 0.5 2 P0.5T20 10 0.5 20 P1.0T0 10 1.0 0 P1.0T2 10 1.0 2 P1.0T20 10 1.0 20 表 2 不同条件下的吸附动力学参数
Table 2. Adsorption kinetic parameters under different conditions
试验组别 准一级动力学 准二级动力学 k1/〔g/(mg·min)〕 R2 k2/〔g/(mg·min)〕 R2 P0.013T2 0.26 0.95 0.05 0.98 P0.1T2 0.28 0.98 0.10 1.00 P0.5T2 0.20 0.92 0.07 0.98 P1.0T2 0.30 0.87 0.15 0.94 P0.013T20 0.13 0.94 0.08 0.97 P0.1T20 0.13 0.92 0.08 0.95 P0.5T20 0.76 0.92 0.38 0.97 P1.0T20 0.56 0.95 0.36 0.98 -
[1] 王振红, 李金丽, 严雅萌, 等.纳米二氧化钛对三价砷在大型蚤体内累积与毒性的影响[J]. 环境科学研究,2018,31(6):1123-1128. doi: 10.13198/j.issn.1001-6929.2017.04.06WANG Z H, LI J L, YAN Y M, et al. Influence of nano-TiO2 on accumulation and toxicity of arsenite in Daphnia magna[J]. Research of Environmental Sciences,2018,31(6):1123-1128. doi: 10.13198/j.issn.1001-6929.2017.04.06 [2] 曾晨, 郭少娟, 杨立新.汞、镉、铅、砷单一和混合暴露的毒性效应及机理研究进展[J]. 环境工程技术学报,2018,8(2):221-230. doi: 10.3969/j.issn.1674-991X.2018.02.030ZENG C, GUO S J, YANG L X. Toxic effects and mechanisms of exposure to single and mixture of mercury, cadmium, lead and arsenic[J]. Journal of Environmental Engineering Technology,2018,8(2):221-230. doi: 10.3969/j.issn.1674-991X.2018.02.030 [3] 张道勇, 赵勇胜, 潘响亮.胞外聚合物(EPS)在藻菌生物膜去除污水中Cd的作用[J]. 环境科学研究,2004,17(5):52-55. doi: 10.3321/j.issn:1001-6929.2004.05.014ZHANG D Y, ZHAO Y S, PAN X L. The role of EPS in removing cadmium in sewage by algae-bacteria biofilm[J]. Research of Environmental Sciences,2004,17(5):52-55. doi: 10.3321/j.issn:1001-6929.2004.05.014 [4] 李妍丽. 微型绿藻对砷污染水体的生物修复研究[D]. 广州: 华南理工大学, 2012. [5] LUO Z X, WANG Z H, YAN Y M, et al. Titanium dioxide nanoparticles enhance inorganic arsenic bioavailability and methylation in two freshwater algae species[J]. Environmental Pollution,2018,238:631-637. doi: 10.1016/j.envpol.2018.03.070 [6] JIANG Y, PURCHASE D, JONES H, et al. Technical note: effects of arsenate (As5+) on growth and production of glutathione (GSH) and phytochelatins (PCS) in Chlorella vulgaris[J]. International Journal of Phytoremediation,2011,13(8):834-844. doi: 10.1080/15226514.2010.525560 [7] ZHANG J Y, NI Y Y, DING T D, et al. The role of humic acid in the toxicity of arsenite to the diatom Navicula sp[J]. Environmental Science and Pollution Research International,2014,21(6):4366-4375. doi: 10.1007/s11356-013-2413-3 [8] NAVARRO E, BAUN A, BEHRA R, et al. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi[J]. Ecotoxicology (London, England),2008,17(5):372-386. doi: 10.1007/s10646-008-0214-0 [9] ROBICHAUD C O, UYAR A E, DARBY M R, et al. Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment[J]. Environmental Science & Technology,2009,43(12):4227-4233. [10] MUELLER N C, NOWACK B. Exposure modeling of engineered nanoparticles in the environment[J]. Environmental Science & Technology,2008,42(12):4447-4453. [11] GOTTSCHALK F, SUN T Y, NOWACK B. Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies[J]. Environmental Pollution,2013,181:287-300. doi: 10.1016/j.envpol.2013.06.003 [12] GONDIKAS A P, von der KAMMER F, REED R B, et al. Release of TiO2 nanoparticles from sunscreens into surface waters: a one-year survey at the old Danube Recreational Lake[J]. Environmental Science & Technology,2014,48(10):5415-5422. [13] SUN H W, ZHANG X Z, ZHANG Z Y, et al. Influence of titanium dioxide nanoparticles on speciation and bioavailability of arsenite[J]. Environmental Pollution,2009,157(4):1165-1170. doi: 10.1016/j.envpol.2008.08.022 [14] LUO Z X, LI M T, WANG Z H, et al. Effect of titanium dioxide nanoparticles on the accumulation and distribution of arsenate in Daphnia magna in the presence of an algal food[J]. Environmental Science and Pollution Research International,2018,25(21):20911-20919. doi: 10.1007/s11356-018-2265-y [15] 杨舒萍, 杨帆, 董四君, 等.纳米二氧化钛增强五价砷对丰年虾的慢性毒性作用[J]. 环境科学研究,2021,34(12):3002-3011. doi: 10.13198/j.issn.1001-6929.2021.09.04YANG S P, YANG F, DONG S J, et al. Titanium dioxide nanoparticles enhanced chronic toxic effects of arsenate in Artemia salina[J]. Research of Environmental Sciences,2021,34(12):3002-3011. doi: 10.13198/j.issn.1001-6929.2021.09.04 [16] YANG F, ZENG L Q, LUO Z X, et al. Complex role of titanium dioxide nanoparticles in the trophic transfer of arsenic from Nannochloropsis maritima to Artemia salina nauplii[J]. Aquatic Toxicology (Amsterdam, Netherlands),2018,198:231-239. doi: 10.1016/j.aquatox.2018.03.009 [17] KELLER A A, WANG H T, ZHOU D X, et al. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices[J]. Environmental Science & Technology,2010,44(6):1962-1967. [18] ZHANG S Y, RENSING C, ZHU Y G. Cyanobacteria-mediated arsenic redox dynamics is regulated by phosphate in aquatic environments[J]. Environmental Science & Technology,2014,48(2):994-1000. [19] 张金羽, 陈双双, 唐林茜, 等.莱茵衣藻对砷酸盐的富集分配和形态转化[J]. 环境化学,2021,40(6):1847-1854. doi: 10.7524/j.issn.0254-6108.2020020703ZHANG J Y, CHEN S S, TANG L X, et al. Accumulation, distribution and transformation of arsenate by Chlamydomonas reinhardtii[J]. Environmental Chemistry,2021,40(6):1847-1854. doi: 10.7524/j.issn.0254-6108.2020020703 [20] LORENC W, KRUSZKA D, KACHLICKI P, et al. Arsenic species and their transformation pathways in marine plants. usefulness of advanced hyphenated techniques HPLC/ICP-MS and UPLC/ESI-MS/MS in arsenic species analysis[J]. Talanta,2020,220:121384. doi: 10.1016/j.talanta.2020.121384 [21] BRUNELLI A, POJANA G, CALLEGARO S, et al. Agglomeration and sedimentation of titanium dioxide nanoparticles (n-TiO2) in synthetic and real waters[J]. Journal of Nanoparticle Research,2013,15(6):1-10. [22] LI L, SILLANPÄÄ M, RISTO M. Influences of water properties on the aggregation and deposition of engineered titanium dioxide nanoparticles in natural waters[J]. Environmental Pollution,2016,219:132-138. doi: 10.1016/j.envpol.2016.09.080 [23] CHEN L Z, ZHOU L N, LIU Y D, et al. Toxicological effects of nanometer titanium dioxide (nano-TiO2) on Chlamydomonas reinhardtii[J]. Ecotoxicology and Environmental Safety,2012,84:155-162. doi: 10.1016/j.ecoenv.2012.07.019 [24] ZHANG S, DENG R, LIN D H, et al. Distinct toxic interactions of TiO2 nanoparticles with four coexisting organochlorine contaminants on algae[J]. Nanotoxicology,2017,11(9/10):1115-1126. [25] WAGLE D, SHIPLEY H J. Adsorption of arsenic (Ⅴ) to titanium dioxide nanoparticles: effect of particle size, solid concentration, and other metals[J]. Environmental Engineering Science,2016,33(5):299-305. doi: 10.1089/ees.2016.0014 [26] LI Y, CAI X J, GUO J W, et al. UV-induced photoactive adsorption mechanism of arsenite by anatase TiO2 with high surface hydroxyl group density[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects,2014,462:202-210. [27] JI J, LONG Z F, LIN D H. Toxicity of oxide nanoparticles to the green algae Chlorella sp.[J]. Chemical Engineering Journal,2011,170(2/3):525-530. [28] LAVRINOVIČS A, MEŽULE L, JUHNA T. Microalgae starvation for enhanced phosphorus uptake from municipal wastewater[J]. Algal Research,2020,52:102090. doi: 10.1016/j.algal.2020.102090 [29] GUPTA K, BHATTACHARYA S, CHATTOPADHYAY D, et al. Ceria associated manganese oxide nanoparticles: synthesis, characterization and arsenic(Ⅴ) sorption behavior[J]. Chemical Engineering Journal,2011,172(1):219-229. doi: 10.1016/j.cej.2011.05.092 [30] WANG N X, LI Y, DENG X H, et al. Toxicity and bioaccumulation kinetics of arsenate in two freshwater green algae under different phosphate regimes[J]. Water Research,2013,47(7):2497-2506. doi: 10.1016/j.watres.2013.02.034 [31] BUTTON D K, DUNKER S S, MORSE M L. Continuous culture of Rhodotorula rubra: kinetics of phosphate-arsenate uptake, inhibition, and phosphate-limited growth[J]. Journal of Bacteriology,1973,113(2):599-611. doi: 10.1128/jb.113.2.599-611.1973 [32] WANG Y, ZHANG C H, ZHENG Y H, et al. Bioaccumulation kinetics of arsenite and arsenate in Dunaliella salina under different phosphate regimes[J]. Environmental Science and Pollution Research International,2017,24(26):21213-21221. doi: 10.1007/s11356-017-9758-y [33] 谢晓玲, 周蓉, 邓自发.光、温限制后铜绿微囊藻和斜生栅藻的超补偿生长与竞争效应[J]. 生态学报,2014,34(5):1224-1234.XIE X L, ZHOU R, DENG Z F. Overcompensation and competitive effects of Microcystis aeruginosa and Scenedesmus obliquus after low temperature and light stresses[J]. Acta Ecologica Sinica,2014,34(5):1224-1234. [34] LUO Z X, WANG Z H, YAN Y M, et al. Methods of determining titanium dioxide nanoparticles enhance inorganic arsenic bioavailability and methylation in two freshwater algae species[J]. MethodsX,2018,5:620-625. doi: 10.1016/j.mex.2018.06.004 [35] ZHANG J Y, ZHOU F, LIU Y X, et al. Effect of extracellular polymeric substances on arsenic accumulation in Chlorella pyrenoidosa[J]. Science of the Total Environment,2020,704:135368. doi: 10.1016/j.scitotenv.2019.135368 [36] SUN J, CHENG J, YANG Z B, et al. Microstructures and functional groups of Nannochloropsis sp. cells with arsenic adsorption and lipid accumulation[J]. Bioresource Technology,2015,194:305-311. doi: 10.1016/j.biortech.2015.07.041 [37] DEO R P, SONGKASIRI W, RITTMANN B E, et al. Surface complexation of Neptunium(Ⅴ) onto whole cells and cell components of Shewanella alga: modeling and experimental study[J]. Environmental Science & Technology,2010,44(13):4930-4935. [38] 高旋. 胞外高聚物对藻细胞和TiO2纳米颗粒界面作用的影响及机制[D]. 杭州: 浙江大学, 2021. [39] 李金丽, 李梦婷, 黄兵, 等.亚砷酸盐提高藻与蚤培养基下纳米二氧化钛的稳定性[J]. 农业环境科学学报,2017,36(2):376-381. doi: 10.11654/jaes.2016-1031LI J L, LI M T, HUANG B, et al. Arsenite enhance the stability of nano-TiO2 in aquatic culture media for freshwater algae and daphnia[J]. Journal of Agro-Environment Science,2017,36(2):376-381. doi: 10.11654/jaes.2016-1031 [40] LI X Y, PAN J F, LU Z Y, et al. Arsenate toxicity to the marine microalga Chlorella vulgaris increases under phosphorus-limited condition[J]. Environmental Science and Pollution Research International,2021,28(36):50908-50918. doi: 10.1007/s11356-021-14318-2 [41] WANG Z H, LUO Z X, YAN C Z, et al. Arsenic uptake and depuration kinetics in Microcystis aeruginosa under different phosphate regimes[J]. Journal of Hazardous Materials,2014,276:393-399. doi: 10.1016/j.jhazmat.2014.05.049 [42] XUE X M, YE J, RABER G, et al. Arsenic methyltransferase is involved in arsenosugar biosynthesis by providing DMA[J]. Environmental Science & Technology,2017,51(3):1224-1230. [43] MIYASHITA S, FUJIWARA S, TSUZUKI M, et al. Rapid biotransformation of arsenate into oxo-arsenosugars by a freshwater unicellular green alga, Chlamydomonas reinhardtii[J]. Bioscience, Biotechnology, and Biochemistry,2011,75(3):522-530. doi: 10.1271/bbb.100751 [44] ENDER E, SUBIRANA M A, RAAB A, et al. Why is NanoSIMS elemental imaging of arsenic in seaweed (Laminaria digitata) important for understanding of arsenic biochemistry in addition to speciation information[J]. Journal of Analytical Atomic Spectrometry,2019,34(11):2295-2302. doi: 10.1039/C9JA00187E [45] CHEN F R, XIAO Z G, YUE L, et al. Algae response to engineered nanoparticles: current understanding, mechanisms and implications[J]. Environmental Science:Nano,2019,6(4):1026-1042. doi: 10.1039/C8EN01368C [46] XUE X M, YE J, RABER G, et al. Identification of steps in the pathway of arsenosugar biosynthesis[J]. Environmental Science & Technology,2019,53(2):634-641. [47] GLABONJAT R A, BLUM J S, MILLER L G, et al. Arsenolipids in cultured Picocystis strain ML and their occurrence in biota and sediment from Mono Lake, California[J]. Life (Basel, Switzerland),2020,10(6):93. [48] GUO P R, GONG Y, WANG C, et al. Arsenic speciation and effect of arsenate inhibition in a Microcystis aeruginosa culture medium under different phosphate regimes[J]. Environmental Toxicology and Chemistry,2011,30(8):1754-1759. doi: 10.1002/etc.567 [49] HELLWEGER F L, FARLEY K J, LALL U, et al. Greedy algae reduce arsenate[J]. Limnology and Oceanography,2003,48(6):2275-2288. doi: 10.4319/lo.2003.48.6.2275 [50] LEVY J L, STAUBER J L, ADAMS M S, et al. Toxicity, biotransformation, and mode of action of arsenic in two freshwater microalgae (Chlorella sp. and Monoraphidium arcuatum)[J]. Environmental Toxicology and Chemistry,2005,24(10):2630-2639. doi: 10.1897/04-580R.1 [51] CHEN Q Q, HU X L, YIN D Q, et al. Effect of subcellular distribution on nC60 uptake and transfer efficiency from Scenedesmus obliquus to Daphnia magna[J]. Ecotoxicology and Environmental Safety,2016,128:213-221. doi: 10.1016/j.ecoenv.2016.02.026 [52] GEITNER N K, MARINAKOS S M, GUO C, et al. Nanoparticle surface affinity as a predictor of trophic transfer[J]. Environmental Science & Technology,2016,50(13):6663-6669. ⊗ -





 下载:
下载: