Influence mechanism of amide-based amphoteric molecules on uranium adsorption capacity of WS2
-
摘要:
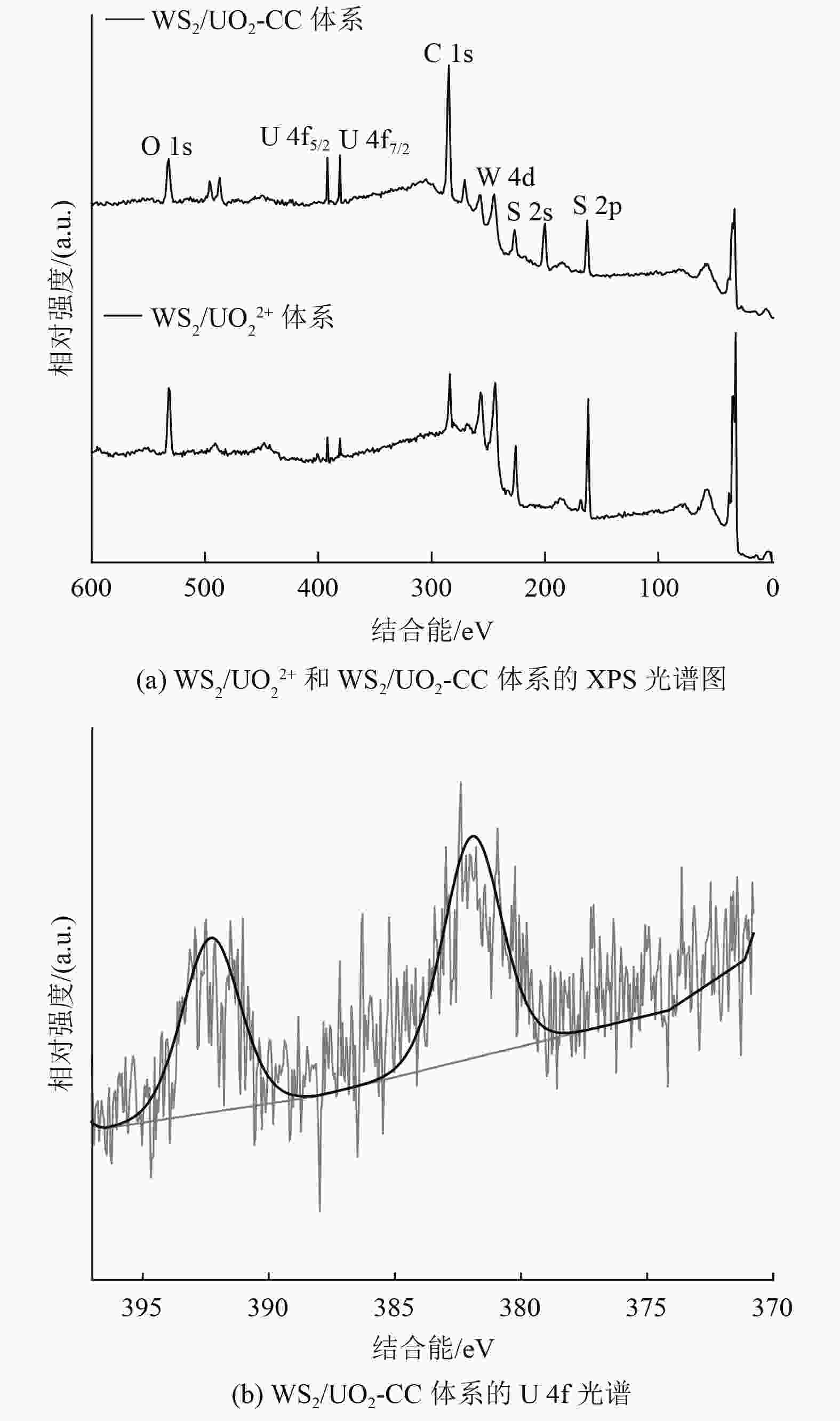
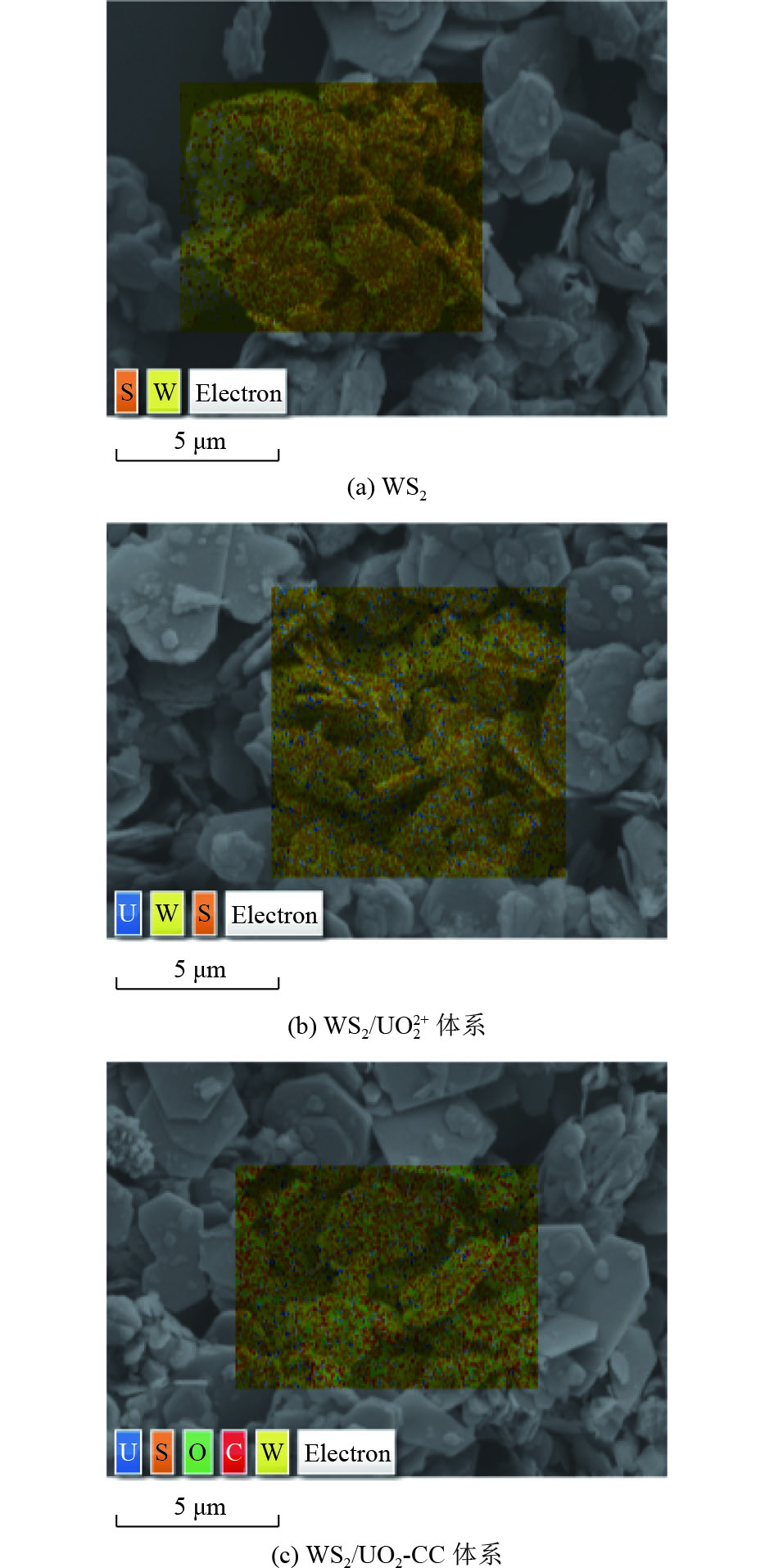
含铀废水中铀的回收主要是基于材料与[UO2(H2O)5]2+中UO2 2+之间的络合,但H2O的电偶极矩对络合有显著弱化作用。采用酰胺基两性分子N,N-二甲基-9-癸烯酰胺(NADA)氢键作用与[UO2(H2O)5]2+形成UO2[(H2O)xC12H23NO]n *(x<5,UO2-Coordination Compound,简称UO2-CC),选取惰性物质WS2为吸附材料,通过静态吸附试验(不同pH、接触时间、浓度和温度)分别研究其对UO2 2+和UO2-CC的吸附量。动力学拟合结果表明,二者的吸附反应是化学吸附过程,UO2 2+经NADA重构后,吸附时间从240 min缩短至180 min,准二级动力学吸附常数提高1.35倍。等温吸附研究结果表明,WS2与UO2-CC络合过程符合Langmuir吸附等温模型,且NADA的加入使吸附由自发吸热过程转变为自发放热过程,吸附反应过程有序度增加。NADA原位重构[UO2(H2O)5]2+后,WS2对UO2 2+的平衡吸附量由45.03 mg/g(WS2/UO2 2+体系)提高到122.14 mg/g(WS2/UO2-CC体系)。采用光谱分析(X射线光电子能谱法)从分子水平深入研究NADA原位重构[UO2(H2O)5]2+后在WS2上的吸附机制,揭示静电、氢键和U—S共价键等作用力对吸附的贡献,特别是NADA的氢键作用。
-
关键词:
- 铀酰离子(UO2 2+) /
- 两性分子 /
- 铀酰活性位点 /
- WS2纳米片 /
- 络合机理
Abstract:The recovery of uranium from uranium-containing wastewater is mainly based on the complexation between the material and UO2 2+ in [UO2(H2O)5]2+, but the electric dipole moment of H2O has a significant weakening effect on the complexation. The amide-based amphoteric molecule N,N-dimethyl-9-decenyl amide (NADA) was used for hydrogen bonding with [UO2(H2O)5]2+ to form UO2[(H2O)xC12H23NO]n * (x<5, UO2-Coordination Compound was named UO2-CC) ,Inert tungsten disulfide (WS2) was selected as the adsorption material, and the adsorption capacities of UO2 2+ and UO2-CC were studied by static adsorption experiments (different pH, contact time, concentration and temperature). The kinetic fitting results showed that the adsorption reaction was a chemisorption process. After NADA reconstruction, the adsorption time of UO2 2+ was shortened from 240 min to 180 min, and the quasi-second-order kinetic adsorption constant was increased by 1.35 times. The results of the isothermal adsorption study showed that the complexation process of WS2 and UO2-CC conformed to the Langmuir adsorption isothermal model, and the addition of NADA made the adsorption change from spontaneous endothermic process to spontaneous exothermic process, and the order degree of the adsorption reaction process increased. After in situ reconstruction of [UO2(H2O)5]2+ by NADA, the equilibrium adsorption capacity of UO2 2+ by WS2 increased from 45.03 mg/g (WS2/UO2 2+ system) to 122.14 mg/g (WS2/UO2-CC system). Spectroscopic analysis by X-ray photoelectron spectroscopy was used to deeply study the adsorption mechanism of NADA on WS2 after in situ reconstruction of [UO2(H2O)5]2+ at the molecular level, and to reveal the contribution of various forces (electrostatic, hydrogen bond and U-S covalent bond) to adsorption, especially the hydrogen bond of NADA.
-
表 1 WS2、WS2/UO22+和WS2/UO2-CC体系中各元素含量
Table 1. Contents of elements in WS2, WS2/UO22+ and WS2/UO2-CC system
% 体系 C N O S W U 合计 WS2 15.57 84.43 100 WS2/UO22+ 14.35 84.06 1.59 100 WS2/UO2-CC 2.00 0.24 0.74 14.48 79.65 2.89 100 表 2 WS2/UO2 2+和WS2/UO2-CC体系的准一级动力学、准二级动力学参数
Table 2. Pseudo-first-order and Pseudo-second-order kinetic parameters of WS2/UO2 2+ and WS2/UO2-CC system
吸附体系 qe/(mg/g) 准一级动力学 准二级动力学 q1/(mg/g) k1/min-1 R2 q2/(mg/g) k2/〔mg/(g·min)〕 R2 WS2/UO2 2+ 46.10 28.83 0.009 0 0.961 47.64 0.000 846 0.997 4 WS2/UO2-CC 122.20 45.90 0.014 7 0.988 123.94 0.001 140 0.999 7 表 3 WS2/UO2 2+和WS2/UO2-CC体系的Langmuir吸附等温模型、Freundlich吸附等温模型参数
Table 3. Langmuir adsorption isothermal model and Freundlich adsorption isothermal model parameters of WS2/UO2 2+ and WS2/UO2-CC system
吸附体系 Langmuir模型 Freundlich模型 KL qm/(mg/g) R2 KF n R2 WS2/UO2 2+ 0.027 37.40 0.985 5.23 1.91 0.974 WS2/UO2-CC 0.018 129.86 0.997 20.33 2.21 0.922 表 4 WS2/UO2 2+和WS2/UO2-CC体系的D-R吸附等温模型参数
Table 4. D-R adsorption isothermal model parameters of WS2/UO2 2+ and WS2/UO2-CC system
吸附体系 qD-R/(mg/g) β/(mol2/kJ2) ED-R/(kJ/mol) R2 WS2/UO2 2+ 0.001 3 0.004 8 10.24 0.982 WS2/UO2-CC 0.003 5 0.004 6 10.47 0.934 注:ED-R为吸附平均自由能,kJ/mol。$E_{ {\rm{D} }{\text -}{\rm{R} } }=1/\sqrt{2\beta}$。 表 5 WS2/UO2 2+和WS2/UO2-CC体系的热力学参数
Table 5. Thermodynamic parameters of WS2/UO2 2+ system and WS2/UO2-CC system
吸附体系 ΔG/(kJ/mol) ΔH/(kJ/mol) ΔS/〔J/(mol·K)〕 278.15 K 288.15 K 198.15 K 308.15 K 318.15 K WS2/UO2 2+ −14.45 −15.47 −16.50 −17.53 −18.55 14.08 102.50 WS2/UO2-CC −18.82 −19.46 −20.09 −20.73 −21.36 −1.16 63.50 -
[1] 稂涛, 胡南, 张辉, 等.博落回对不同化学形态铀的富集特征[J]. 环境科学研究,2017,30(8):1238-1245.LANG T, HU N, ZHANG H, et al. Accumulation of different chemical species of uranium in Macleaya cordata[J]. Research of Environmental Sciences,2017,30(8):1238-1245. [2] YAN X, YANG L, ZHANG X, et al. Concept of an accelerator-driven advanced nuclear energy system[J]. Energies,2017,10(7):944-957. doi: 10.3390/en10070944 [3] LADSHAW A P, IVANOV A S, DAS S, et al. First-principles integrated adsorption modeling for selective capture of uranium from seawater by polyamidoxime sorbent materials[J]. ACS Applied Materials & Interfaces,2018,10(15):12580-12593. [4] 律志民, 杨世民, 陈磊, 等.新型LDH@MOF-76复合材料对于水溶液中铀酰的高效富集[J]. 中国科学:化学,2019,49(1):53-64. doi: 10.1360/N032018-00112LÜ Z M, YANG S M, CHEN L, et al. Enhanced removal of uranium(Ⅵ) from aqueous solution by a novel LDH@MOF-76 composite[J]. Scientia Sinica Chimica),2019,49(1):53-64. doi: 10.1360/N032018-00112 [5] WANG Z M, LEE S W, CATALANO J G, et al. Adsorption of uranium(Ⅵ) to manganese oxides: X-ray absorption spectroscopy and surface complexation modeling[J]. Environmental Science & Technology,2013,47(2):850-858. [6] LI Z J, CHEN F, YUAN L Y, et al. Uranium(Ⅵ) adsorption on graphene oxide nanosheets from aqueous solutions[J]. Chemical Engineering Journal,2012,210:539-546. doi: 10.1016/j.cej.2012.09.030 [7] HAN R P, ZOU W H, WANG Y, et al. Removal of uranium(Ⅵ) from aqueous solutions by manganese oxide coated zeolite: discussion of adsorption isotherms and pH effect[J]. Journal of Environmental Radioactivity,2007,93(3):127-143. doi: 10.1016/j.jenvrad.2006.12.003 [8] FASFOUS I I, DAWOUD J N. Uranium(Ⅵ) sorption by multiwalled carbon nanotubes from aqueous solution[J]. Applied Surface Science,2012,259:433-440. doi: 10.1016/j.apsusc.2012.07.062 [9] BACHMAF S, MERKEL B J. Sorption of uranium(Ⅵ) at the clay mineral–water interface[J]. Environmental Earth Sciences,2011,63(5):925-934. doi: 10.1007/s12665-010-0761-6 [10] ABD EL-MAGIED M O. Sorption of uranium ions from their aqueous solution by resins containing nanomagnetite particles[J]. Journal of Engineering,2016,2016:7214348. [11] SINGH S, KAUR M, BAJWA B S, et al. Salicylaldehyde and 3-hydroxybenzoic acid grafted NH2-MCM-41: synthesis, characterization and application as U(Ⅵ) scavenging adsorbents using batch mode, column and membrane systems[J]. Journal of Molecular Liquids,2022,346:117061. doi: 10.1016/j.molliq.2021.117061 [12] JIANG X Y, WANG H Q, WANG Q L, et al. Immobilizing amino-functionalized mesoporous silica into sodium alginate for efficiently removing low concentrations of uranium[J]. Journal of Cleaner Production,2020,247:119162. doi: 10.1016/j.jclepro.2019.119162 [13] BARBER P S, KELLEY S P, GRIGGS C, et al. Surface modification of ionic liquid-spun chitin fibers for the extraction of uranium from seawater: seeking the strength of chitin and the chemical functionality of chitosan[J]. Green Chemistry,2014,16:1828-1836. doi: 10.1039/C4GC00092G [14] OMICHI H, KATAKAI A, SUGO T, et al. A new type of amidoxime-group-containing adsorbent for the recovery of uranium from seawater[J]. Separation Science and Technology,1985,20(2/3):163-178. [15] 汤家喜, 朱永乐, 刘悦, 等.生物炭对农业面源污染物中农药分子的吸附性能研究[J]. 环境工程技术学报,2020,10(6):1057-1062.TANG J X, ZHU Y L, LIU Y, et al. Research on adsorption properties of biochar for pesticide molecules of agricultural non-point source pollutants[J]. Journal of Environmental Engineering Technology,2020,10(6):1057-1062. [16] SEN N, DAREKAR M, SIRSAT P, et al. Recovery of uranium from lean streams by extraction and direct precipitation in microchannels[J]. Separation and Purification Technology,2019,227:115641. doi: 10.1016/j.seppur.2019.05.083 [17] ZHAO G X, LI J X, REN X M, et al. Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management[J]. Environmental Science & Technology,2011,45(24):10454-10462. [18] ROMANCHUK A Y, SLESAREV A S, KALMYKOV S N, et al. Graphene oxide for effective radionuclide removal[J]. Physical Chemistry Chemical Physics:PCCP,2013,15(7):2321-2327. doi: 10.1039/c2cp44593j [19] 石万里, 赵泽华, 叶飞, 等.酸改性凹凸棒土处理含铜废水的试验研究[J]. 环境工程技术学报,2018,8(2):169-175.SHI W L, ZHAO Z H, YE F, et al. Experimental study on adsorption of copper containing wastewater by modified attapulgite[J]. Journal of Environmental Engineering Technology,2018,8(2):169-175. [20] KUCHERENKO M, IZMODENOVA S V, KRUCHININ N, et al. Change in the kinetics of delayed annihilation fluorescence during rearrangement of polymer-chain structure in a nanocavity of a solid adsorbent[J]. High Energy Chemistry,2009,43:592-598. doi: 10.1134/S0018143909070169 [21] 王洁. 多巴胺化组装体的构建、功能化及应用[D]. 上海: 上海交通大学, 2016. [22] ZHANG X H, LEI W N, YE X, et al. A facile synthesis and characterization of graphene-like WS2 nanosheets[J]. Materials Letters,2015,159:399-402. doi: 10.1016/j.matlet.2015.07.044 [23] SAVVIN S B. Analytical use of arsenazo Ⅲ: determination of thorium, zirconium, uranium and rare earth elements[J]. Talanta,1961,8(9):673-685. doi: 10.1016/0039-9140(61)80164-1 [24] ZHANG Z, DONG Z, DAI Y, et al. Amidoxime-functionalized hydrothermal carbon materials for uranium removal from aqueous solution[J]. RSC Advances,2016,6(104):102462-102471. doi: 10.1039/C6RA21986A [25] SINGH S, BAJWA B S, KAUR I. (Zn/Co)-zeolitic imidazolate frameworks: room temperature synthesis and application as promising U(Ⅵ) scavengers: a comparative study[J]. Journal of Industrial and Engineering Chemistry,2021,93:351-360. doi: 10.1016/j.jiec.2020.10.012 [26] 史济斌, 刘国杰.评Freundlich吸附等温式的推导[J]. 大学化学,2015,30(3):76-79. doi: 10.3866/pku.DXHX2010376SHI J B, LIU G J. The derivation of freundlich adsorption isotherm[J]. University Chemistry,2015,30(3):76-79. doi: 10.3866/pku.DXHX2010376 [27] SHARMA S, BHAGAT S, SINGH J, et al. Temperature dependent photoluminescence from WS2 nanostructures[J]. Journal of Materials Science:Materials in Electronics,2018,29(23):20064-20070. doi: 10.1007/s10854-018-0137-3 [28] HAZARIKA S J, MOHANTA D. Inorganic fullerene-type WS2 nanoparticles: processing, characterization and its photocatalytic performance on malachite green[J]. Applied Physics A,2017,123(5):1-10. [29] 田晓冬, 前田宁.N-异丙基丙烯酰胺共聚物的合成及温敏性[J]. 高分子材料科学与工程,2010,26(4):29-32.TIAN X D, QIAN T N. Synthesis and thermo-sensitive research of N-isopropylacrylamide copolymers[J]. Polymer Materials Science & Engineering,2010,26(4):29-32. [30] 刘彦静, 曾小兵, 代朝猛, 等.石墨烯纳米复合材料在水处理中的应用研究进展[J]. 材料导报,2013,27(7):127-130.LIU Y J, ZENG X B, DAI C M, et al. Progress in the applied research of graphene nanocomposites in water treatment[J]. Materials Review,2013,27(7):127-130. [31] SONG W C, WANG X X, WANG Q, et al. Plasma-induced grafting of polyacrylamide on graphene oxide nanosheets for simultaneous removal of radionuclides[J]. Physical Chemistry Chemical Physics:PCCP,2015,17(1):398-406. doi: 10.1039/C4CP04289A [32] BAYRAMOĞLU G, ÇELIK G, ARICA M Y. Studies on accumulation of uranium by fungus Lentinus sajor-caju[J]. Journal of Hazardous Materials,2006,136(2):345-353. doi: 10.1016/j.jhazmat.2005.12.027 [33] LIANG X F, XU Y M, WANG L, et al. Sorption of Pb2+ on mercapto functionalized sepiolite[J]. Chemosphere,2013,90(2):548-555. doi: 10.1016/j.chemosphere.2012.08.027 [34] LIU S J. Cooperative adsorption on solid surfaces[J]. Journal of Colloid and Interface Science,2015,450:224-238. doi: 10.1016/j.jcis.2015.03.013 [35] WANG F H, LIU Q, LI R M, et al. Selective adsorption of uranium(Ⅵ) onto prismatic sulfides from aqueous solution[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects,2016,490:215-221. doi: 10.1016/j.colsurfa.2015.11.045 [36] MANOS M J, KANATZIDIS M G. Layered metal sulfides capture uranium from seawater[J]. Journal of the American Chemical Society,2012,134(39):16441-16446. doi: 10.1021/ja308028n [37] DOU W X, YANG W T, ZHAO X J, et al. Hollow cobalt sulfide for highly efficient uranium adsorption from aqueous solutions[J]. Inorganic Chemistry Frontiers,2019,6(11):3230-3236. ⊗ doi: 10.1039/C9QI00737G -





 下载:
下载: