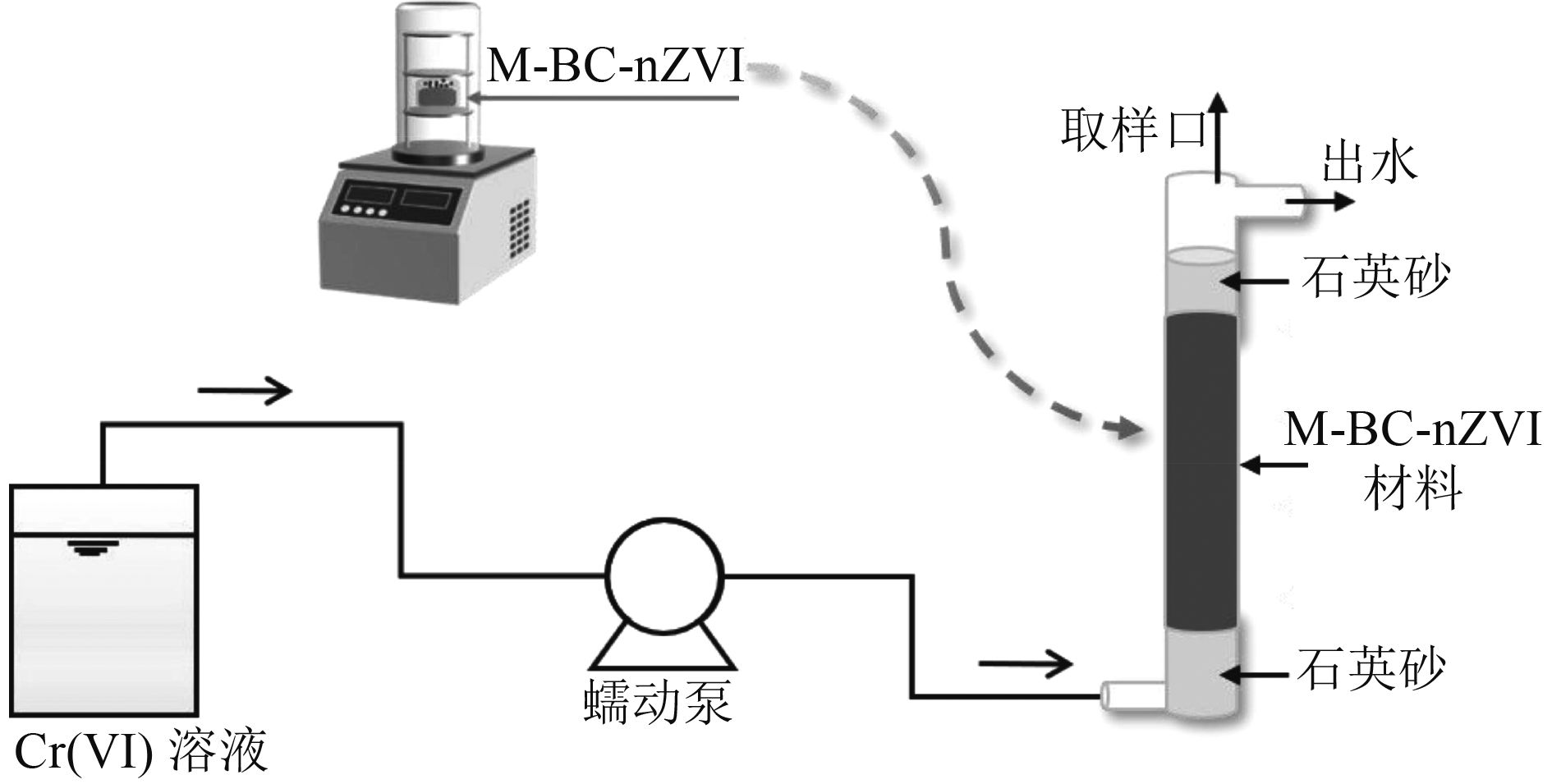
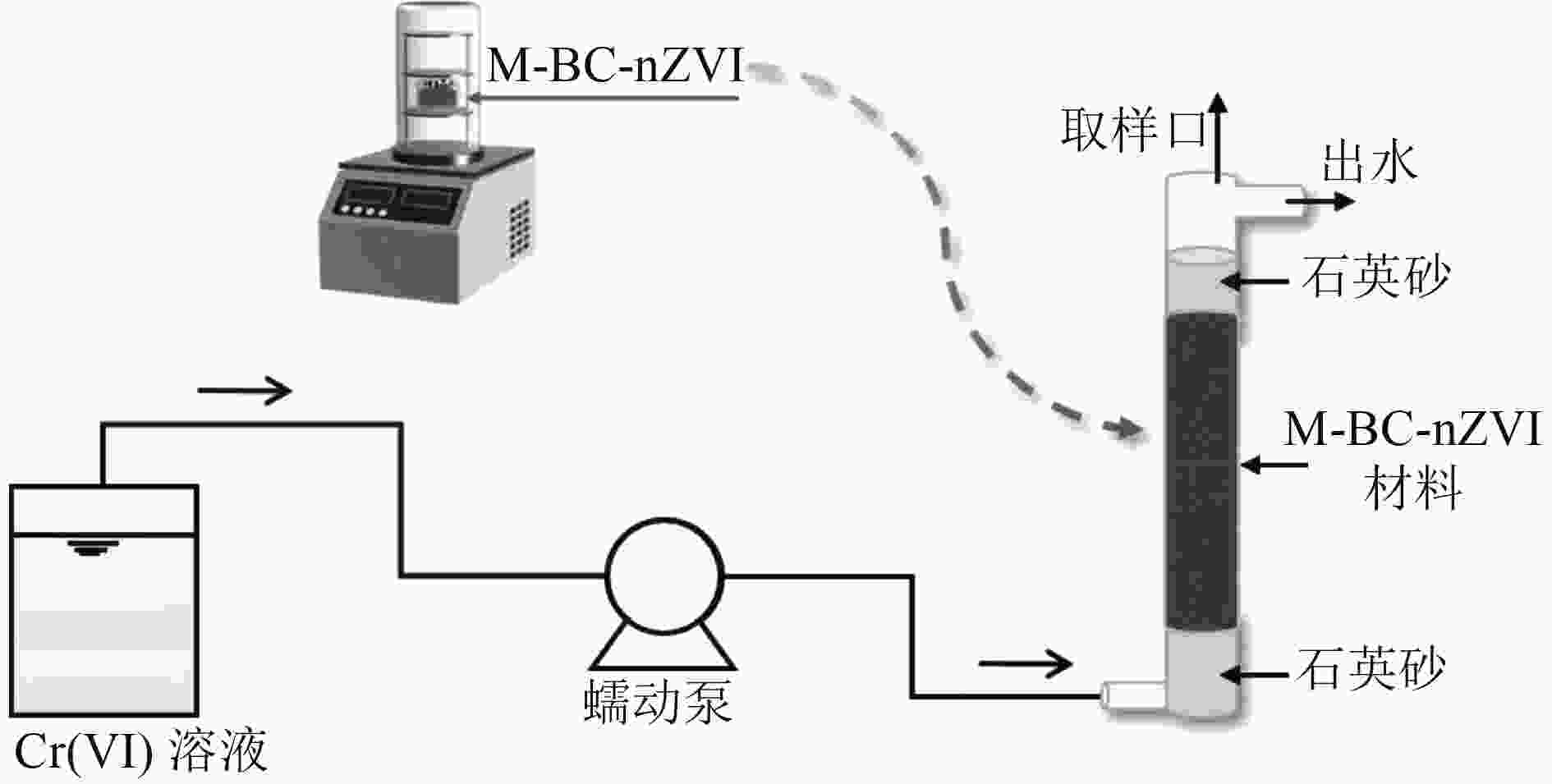
Experimental study on the removal of Cr(Ⅵ) from water by biochar-based sulfide modification loaded with nano-zero valent iron
-
摘要:
为研发治理地下水Cr(Ⅵ)污染的可行除铬材料,以碳热法制得生物炭负载纳米零价铁(BC-nZVI),并通过对BC-nZVI硫化改性制备得到改性材料(M-BC-nZVI),采用除铬容量、铬铁比(Cr/Fe)、反应活性分析M-BC-nZVI的除铬优势,通过模拟柱试验建立失效速率模型,从而推算M-BC-nZVI完全失效的除铬容量,最后与相关文献数据进行对比,分析M-BC-nZVI除Cr(Ⅵ)的应用可行性。结果表明:M-BC-nZVI材料的除铬容量、Cr/Fe、拟一级反应速率常数(kobs)分别是BC-nZVI的1.86倍、1.95倍和3.00倍,因此相对于BC-nZVI来说M-BC-nZVI更具除铬优势;各模拟柱在运行过程中无明显堵塞情况,且随着进水浓度的升高,M-BC-nZVI的失效速率常数变大。当失效除铬速率为初始除铬速率的1.0%、进水Cr(Ⅵ)浓度为5 mg/L时,除铬容量最高,可以达到12.70 mg/g;对比M-BC-nZVI与其他文献报道的铁基材料及铁基改性材料的Cr/Fe可知,M-BC-nZVI的Cr/Fe为其他文献的1.06~42.06倍,故从材料的除铬性能来看,M-BC-nZVI应用于可渗透反应墙处理地下水Cr(Ⅵ)污染可行。
Abstract:In order to treat Cr(Ⅵ) pollution in groundwater, biochar-supported nano-zero valent iron (BC-nZVI) was prepared by the carbothermal method, and the modified material (M-BC-nZVI) was prepared by vulcanization modification of BC-nZVI. The chromium removal capacity, Cr to Fe ratio (Cr/Fe) and the reactivity of M-BC-nZVI were used to analyze the superiority of M-BC-nZVI for chromium removal. A failure rate model was established through the simulated column test to calculate the chromium removal capacity of M-BC-nZVI that completely failed. Finally, the application feasibility of M-BC-nZVI in removing Cr(Ⅵ) was analyzed by comparing it with the relevant studies. The results showed that the removal capacity, Cr/Fe and pseudo-first-order reaction rate constant (kobs) of M-BC-nZVI were 1.86, 1.95 and 3.00 times higher than those of BC-nZVI, respectively. Therefore, compared with BC-nZVI, M-BC-nZVI had certain advantages in various aspects. Each simulated column had no obvious blockage during operation, and the failure rate constant of M-BC-nZVI increased with the increase of influent concentration. The highest chromium removal capacity (12.70 mg/g) reached when the failure chromium removal rate was 1.0% of the initial chromium removal rate and the influent Cr(Ⅵ) concentration was 5 mg/L. By comparing Cr/Fe of M-BC-nZVI with iron-based materials and iron-based modified materials reported in other studies, Cr/Fe of M-BC-nZVI was 1.06 to 42.06 times that of other studies. Therefore, based on the chromic removal performance of the material, it was feasible to apply M-BC-nZVI to permeable reactive barrier to treat Cr(Ⅵ) pollution in groundwater.
-
Key words:
- biochar /
- nano-zero-valent iron /
- vulcanization modification /
- hexavalent chromium /
- simulated column
-
表 1 模拟柱除铬速率拟合参数
Table 1. Fitting parameters of simulated column chromium removal rate
反应模型 模拟柱 k1/h−1 k2/mg−1 R2 拟一级反应 1号 0.011 6 0.800 6 2号 0.010 9 0.960 6 3号 0.009 0 0.949 0 拟二级反应 1号 0.048 2 0.952 3 2号 0.042 2 0.972 7 3号 0.035 4 0.962 2 表 2 除铬总量与除铬容量计算结果
Table 2. Calculation results of total amount and capacity of chromium removal
(失效除铬速率/初始
除铬速率)/%进水Cr(Ⅵ)浓
度/(mg/L)质量/g 除铬总
量/mg除铬容量/
(mg/g)1.0 15 11.9 135.27 11.37 10 11.0 132.06 12.01 5 11.1 141.02 12.70 2.5 15 11.9 109.42 9.20 10 11.0 111.25 10.11 5 11.1 121.76 10.97 5.0 15 11.9 89.91 7.56 10 11.0 95.44 8.68 5 11.1 106.96 9.64 -
[1] FU F L, MA J, XIE L P, et al. Chromium removal using resin supported nanoscale zero-valent iron[J]. Journal of Environmental Management,2013,128:822-827. doi: 10.1016/j.jenvman.2013.06.044 [2] 徐腾, 南丰, 蒋晓锋, 等.制革场地土壤和地下水中铬污染来源及污染特征研究进展[J]. 土壤学报,2020,57(6):1341-1352.XU T, NAN F, JIANG X F, et al. Progresses in research on sources and characteristics of chromium pollution in soils and groundwater of tannery sites[J]. Acta Pedologica Sinica,2020,57(6):1341-1352. [3] 程政乔,姜杰,杨浈.Cr(Ⅵ)污染地下水电动修复过程中的关键指标监测和分析[J]. 环境工程技术学报,2022,12(3):816-823. doi: 10.12153/j.issn.1674-991X.20210492CHENG Z Q,JIANG J,YANG Z. Monitoring and analysis of key indicators in the process of electrickinetic remediation of Cr(Ⅵ) contaminated groundwater[J]. Journal of Environmental Engineering Technology,2022,12(3):816-823. doi: 10.12153/j.issn.1674-991X.20210492 [4] GAO Y, XIA J. Chromium contamination accident in China: viewing environment policy of China[J]. Environmental Science & Technology,2011,45(20):8605-8606. [5] DAS N, MATHEW L. Chromium pollution and bioremediation: an overview[M]//Environmental pollution. Dordrecht: Springer Netherlands, 2011: 297-321. [6] JOBBY R, JHA P, YADAV A K, et al. Biosorption and biotransformation of hexavalent chromium [Cr(Ⅵ)]: a comprehensive review[J]. Chemosphere,2018,207:255-266. doi: 10.1016/j.chemosphere.2018.05.050 [7] 田仪娟, 晏超群, 程治良, 等.柑桔皮与铬渣共热解毒六价铬[J]. 无机盐工业,2021,53(12):129-134. doi: 10.19964/j.issn.1006-4990.2021-0118TIAN Y J, YAN C Q, CHENG Z L, et al. Detoxification of Cr(Ⅵ) from chromite ore processing residue by pyrolysis with citrus peel[J]. Inorganic Chemicals Industry,2021,53(12):129-134. doi: 10.19964/j.issn.1006-4990.2021-0118 [8] 施周, 贺维鹏.饮用水水源中重金属污染防控技术与对策[J]. 给水排水,2012,48(8):1-3. doi: 10.13789/j.cnki.wwe1964.2012.08.013 [9] 张雨婷. 生物质炭对电镀废水中六价铬的去除及机理研究[D]. 长春: 吉林大学, 2020. [10] 吴进. 磁性聚合物的制备及其同步去除水中铬和砷污染物的研究[D]. 合肥: 合肥工业大学, 2018. [11] CUI J, WANG E D, HOU Z M, et al. Removal of chromium(Ⅵ) from groundwater using oil shale ash supported nanoscaled zero-valent iron[J]. Chemical Research in Chinese Universities,2018,34(4):546-551. doi: 10.1007/s40242-018-8104-3 [12] 王棣, 魏文侠, 王琳玲, 等.纳米铁原位注入技术对六价铬污染地下水的修复[J]. 环境工程学报,2018,12(2):521-526. doi: 10.12030/j.cjee.201706140WANG D, WEI W X, WANG L L, et al. Remediation of chromium(Ⅵ) contaminated groundwater by in situ injection of nanoscale zero valent iron[J]. Chinese Journal of Environmental Engineering,2018,12(2):521-526. doi: 10.12030/j.cjee.201706140 [13] SHAHID M, SHAMSHAD S, RAFIQ M, et al. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review[J]. Chemosphere,2017,178:513-533. doi: 10.1016/j.chemosphere.2017.03.074 [14] 王泓泉.污染地下水可渗透反应墙(PRB)技术研究进展[J]. 环境工程技术学报,2020,10(2):251-259. doi: 10.12153/j.issn.1674-991X.20190129WANG H Q. Study on permeable reactive barrier technology for the remediation of polluted groundwater[J]. Journal of Environmental Engineering Technology,2020,10(2):251-259. doi: 10.12153/j.issn.1674-991X.20190129 [15] 刘美丽, 牛其建, 俞洋洋, 等.碳基材料负载纳米零价铁去除六价铬的研究进展[J]. 环境科学研究,2022,35(3):768-779.LIU M L, NIU Q J, YU Y Y, et al. Progress in removal of hexavalent chromium by carbon-based materials loaded with nano zero-valent iron[J]. Research of Environmental Sciences,2022,35(3):768-779. [16] 孟凡生, 王业耀, 李莉.PRB去除模拟地下水中六价铬的反应特性[J]. 环境工程技术学报,2013,3(2):92-97. doi: 10.3969/j.issn.1674-991X.2013.02.016MENG F S, WANG Y Y, LI L. Reactivity characteristics of hexavalent chromium removed by PRB in simulated ground water[J]. Journal of Environmental Engineering Technology,2013,3(2):92-97. doi: 10.3969/j.issn.1674-991X.2013.02.016 [17] 杨君君, 卢晓霞, 张琪, 等.生物墙对地下水中六价铬的去除效果模拟研究[J]. 环境工程学报,2014,8(11):4568-4574.YANG J J, LU X X, ZHANG Q, et al. Simulated laboratory study on effect of removing chromium(Ⅵ) from groundwater using permeable biowall[J]. Chinese Journal of Environmental Engineering,2014,8(11):4568-4574. [18] 卢欣, 李淼, 唐翠梅, 等.Fe0 -PRB去除Cr(Ⅵ)反应动力学及影响机制[J]. 环境科学,2016,37(9):3473-3479.LU X, LI M, TANG C M, et al. Reaction kinetics and impacting mechanism of Cr(Ⅵ) removal in Fe0-PRB systems[J]. Environmental Science,2016,37(9):3473-3479. [19] QU M, CHEN H X, WANG Y, et al. Improved performance and applicability of copper-iron bimetal by sulfidation for Cr(Ⅵ) removal[J]. Chemosphere,2021,281:130820. doi: 10.1016/j.chemosphere.2021.130820 [20] 朱文会. Cr(Ⅵ)污染地下水修复的PRB填料实验研究[D]. 常州: 常州大学, 2014. [21] MONTESINOS V N, QUICI N, HALAC E B, et al. Highly efficient removal of Cr(Ⅵ) from water with nanoparticulated zerovalent iron: understanding the Fe(Ⅲ)-Cr(Ⅲ) passive outer layer structure[J]. Chemical Engineering Journal,2014,244:569-575. doi: 10.1016/j.cej.2014.01.093 [22] ZHANG B, ZHU B H, WANG X, et al. Nanoscale zero valent iron supported by biomass-activated carbon for highly efficient total chromium removal from electroplating wastewater[J]. Water,2019,12(1):89. doi: 10.3390/w12010089 [23] MANNING B A, KISER J R, KWON H, et al. Spectroscopic investigation of Cr(Ⅲ)- and Cr(Ⅵ)-treated nanoscale zerovalent iron[J]. Environmental Science & Technology,2007,41(2):586-592. [24] JIA Z Z, SHU Y H, HUANG R L, et al. Enhanced reactivity of nZVI embedded into supermacroporous cryogels for highly efficient Cr(Ⅵ) and total Cr removal from aqueous solution[J]. Chemosphere,2018,199:232-242. doi: 10.1016/j.chemosphere.2018.02.021 [25] ALIDOKHT L, KHATAEE A R, REYHANITABAR A, et al. Reductive removal of Cr(Ⅵ) by starch-stabilized Fe0 nanoparticles in aqueous solution[J]. Desalination,2011,270(1/2/3):105-110. ⊗ -




 下载:
下载: