Catalytic combustion performance of copper manganese catalyst for low concentration ethanol
-
摘要:
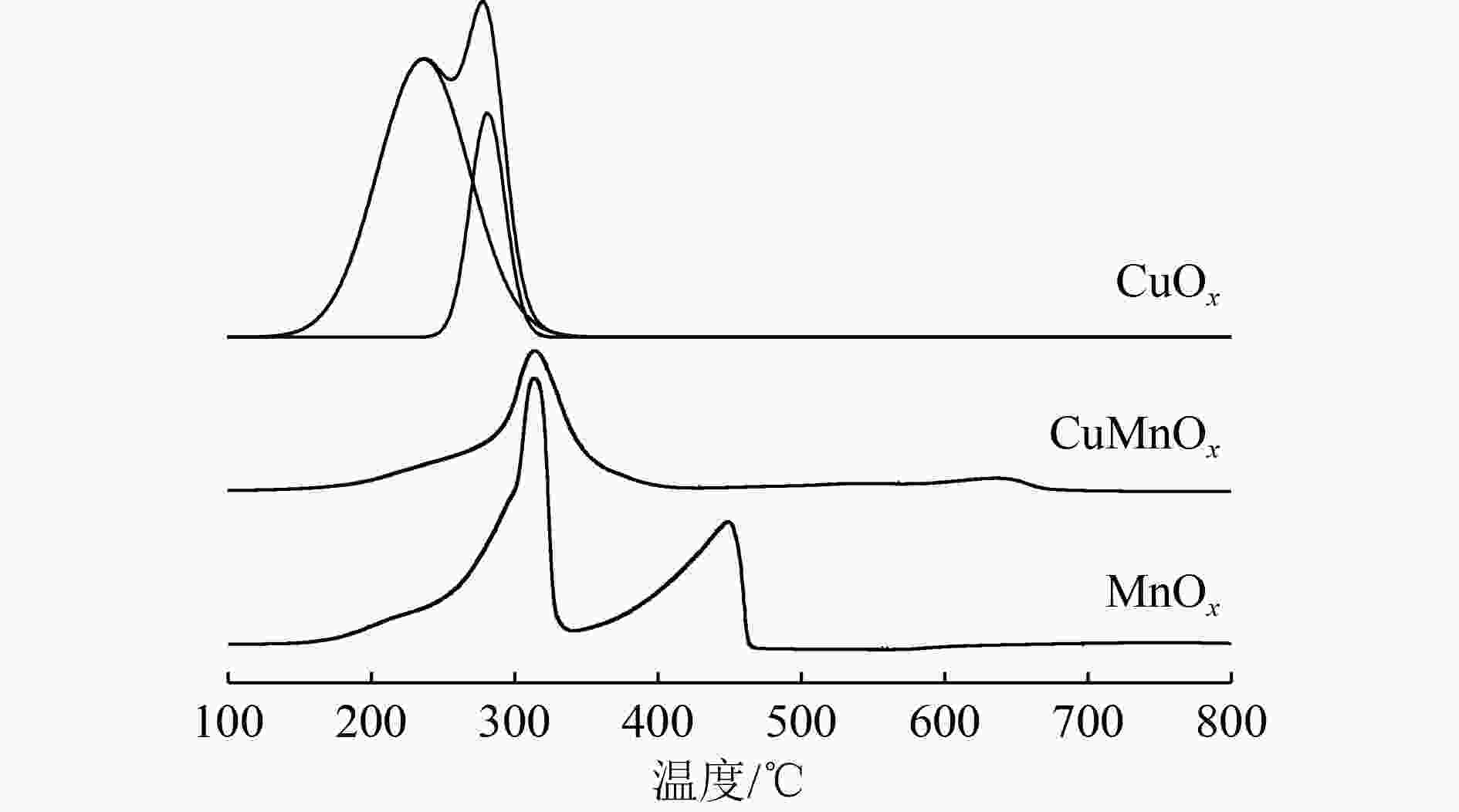
乙醇汽油车尾气中除了传统汽油车的三大常规污染物(CO、NOx和HCs),还含有导致光化学烟雾和臭氧污染的醇醛类非常规有机物。采用共沉淀法制备了CuOx、MnOx和CuMnOx催化剂用于乙醇汽油车冷启动排放的低浓度乙醇的催化燃烧,同时采用氮气吸附脱附技术(BET)、X射线衍射(XRD)、氢气程序升温还原(H2-TPR)、扫描电子显微镜(SEM)、X射线光电子能谱(XPS)对催化剂进行了表征。研究表明,CuMnOx催化剂对低浓度乙醇具有较好的低温催化活性;当反应温度为185 ℃时,CO2产率可达86%,优于单一的CuOx催化剂(约20%);同时,CuMnOx比MnOx具有较高的CO2选择性。Cu、Mn之间的相互作用改变了催化剂的织构、结构和氧化性能,引起了催化剂表面晶貌和电子环境的变化,使得CuMnOx催化剂表面具有大量的氧缺陷位,有利于氧分子在其表面的吸附,进而活化成表面活性氧物种。
Abstract:In addition to the three conventional pollutants (CO, NOx and HCs) of traditional gasoline vehicles, gasohol vehicle exhausts also contained alcohols and aldehydes which led to photochemical smog and ozone pollution. CuOx, MnOx and CuMnOx catalysts were prepared by the co-precipitation method for catalytic combustion of low-concentration ethanol emitted from gasohol vehicles in cold start. Moreover, the catalysts were characterized by BET nitrogen adsorption-desorption, X-ray diffraction (XRD), hydrogen temperature programmed reduction (H2-TPR), scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS). The results showed that CuMnOx catalyst had a better low-temperature catalytic activity for low-concentration ethanol. When the reaction temperature was 185 °C, the yield of CO2 was as high as 86%, better than that of single CuOx catalyst (about 20%). Meanwhile, CuMnOx had a higher CO2 selectivity than MnOx. The interactions between Cu and Mn altered the texture, structure and oxidation performance of the catalyst, resulting in the changes in the crystal morphology and electronic environment of the catalyst. There were a large number of oxygen defect sites on the surface of CuMnOx, which was in favour of the adsorption of oxygen molecules, then turning into surface active oxygen species.
-
表 1 催化剂样品的织构性能
Table 1. Texture properties of the catalyst samples
样品 比表面积/(m2/g) 总孔容/(cm3/g) 平均孔径/nm CuOx 18 0.16 28.6 MnOx 79 0.16 5.5 CuMnOx 47 0.18 7.9 表 2 催化剂样品O1s的XPS数据
Table 2. O1s XPS data of the samples
样品 Oα Oβ Oα/Oβ 能带/eV 相对含量/% 能带/eV 相对含量/% CuOx 531.4 28.77 529.6 71.23 0.40 MnOx 531.4 40.60 529.6 59.40 0.68 CuMnOx 531.4 65.46 529.9 34.54 1.90 -
[1] 吴咏玲. 全国机动车保有量突破4亿辆[EB/OL]. (2022-04-07)[2022-05-04]. http://www.news.cn/2022-04/07/c_1128540220.htm. [2] 纪亮, 袁盈, 李刚, 等.我国机动车排放标准的大气污染物减排效果研究[J]. 环境工程技术学报,2011,1(3):237-242. doi: 10.3969/j.issn.1674-991X.2011.03.039JI L, YUAN Y, LI G, et al. Study on emission reduction effect of motor vehicle emission standards in China[J]. Journal of Environmental Engineering Technology,2011,1(3):237-242. doi: 10.3969/j.issn.1674-991X.2011.03.039 [3] FANG X, SHEN Y, ZHAO J, et al. Status and prospect of lignocellulosic bioethanol production in China[J]. Bioresource Technology,2010,101(13):4814-4819. doi: 10.1016/j.biortech.2009.11.050 [4] 能源局.《关于扩大生物燃料乙醇生产和推广使用车用乙醇汽油的实施方案》印发[A/OL]. (2017-09-13)[2022-05-04]. http://www.gov.cn/xinwen/2017-09/13/content_5224735.htm. [5] 张涛.《2030年前碳达峰行动方案》解读[J]. 生态经济,2022,38(1):9-12. [6] AGARWAL A K, SHUKLA P C, GUPTA J G, et al. Unregulated emissions from a gasohol (E5, E15, M5, and M15) fuelled spark ignition engine[J]. Applied Energy,2015,154:732-741. doi: 10.1016/j.apenergy.2015.05.052 [7] MORKNOY D, KHUMMONGKOL P, PRUEAKSASIT T. Seasonal and diurnal concentrations of ambient formaldehyde and acetaldehyde in Bangkok[J]. Water, Air, & Soil Pollution,2011,216(1/2/3/4):693-702. [8] WANG T, XUE L K, BRIMBLECOMBE P, et al. Ozone pollution in China: a review of concentrations, meteorological influences, chemical precursors, and effects[J]. Science of the Total Environment,2017,575:1582-1596. doi: 10.1016/j.scitotenv.2016.10.081 [9] 曹小聪, 吴晓晨, 徐文帅, 等.三亚市大气VOCs污染特征、臭氧生成潜势及来源解析[J]. 环境科学研究,2021,34(8):1812-1824.CAO X C, WU X C, XU W S, et al. Pollution characterization, ozone formation potential and source apportionment of ambient VOCs in Sanya, China[J]. Research of Environmental Sciences,2021,34(8):1812-1824. [10] SUAREZ-BERTOA R, CLAIROTTE M, ARLITT B, et al. Intercomparison of ethanol, formaldehyde and acetaldehyde measurements from a flex-fuel vehicle exhaust during the WLTC[J]. Fuel,2017,203:330-340. doi: 10.1016/j.fuel.2017.04.131 [11] 沈岩, 武彤冉, 闫静, 等.基于COPERT模型北京市机动车大气污染物和二氧化碳排放研究[J]. 环境工程技术学报,2021,11(6):1075-1082. doi: 10.12153/j.issn.1674-991X.20210289SHEN Y, WU T R, YAN J, et al. Investigation on air pollutants and carbon dioxide emissions from motor vehicles in Beijing based on COPERT model[J]. Journal of Environmental Engineering Technology,2021,11(6):1075-1082. doi: 10.12153/j.issn.1674-991X.20210289 [12] WESTERMANN A, AZAMBRE B, FINQUENEISEL G, et al. Evolution of unburnt hydrocarbons under “cold-start” conditions from adsorption/desorption to conversion: on the screening of zeolitic materials[J]. Applied Catalysis B:Environmental,2014,158/159:48-59. doi: 10.1016/j.apcatb.2014.04.005 [13] CLAIROTTE M, ADAM T W, ZARDINI A A, et al. Effects of low temperature on the cold start gaseous emissions from light duty vehicles fuelled by ethanol-blended gasoline[J]. Applied Energy,2013,102:44-54. doi: 10.1016/j.apenergy.2012.08.010 [14] LIU J Y, ZHAO M, XU C H, et al. Ultrasonic-assisted fabrication and catalytic activity of CeZrAl oxide-supported Pd for the purification of gasohol exhaust[J]. Chinese Journal of Catalysis,2013,34(4):751-757. doi: 10.1016/S1872-2067(11)60488-9 [15] LIU J Y, ZHAO M, XU C H, et al. Hydrothermal synthesis and catalytic activity of CeZrAl oxide-supported Pd for the purification of gasohol exhaust[J]. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry,2015,45(6):899-905. doi: 10.1080/15533174.2013.843554 [16] LIU J Y, LI J J, HOU X X, et al. Thermal stabilities of MCM-41-modified Pd/Al2O3 for ethanol adsorption and oxidation[J]. Industrial & Engineering Chemistry Research,2020,59(13):5474-5481. [17] HASEGAWA Y I, MAKI R U, SANO M, et al. Preferential oxidation of CO on copper-containing manganese oxides[J]. Applied Catalysis A:General,2009,371(1/2):67-72. [18] CHEN H, WANG J H, LI H, et al. Low temperature combustion of ethylene in a carbon dioxide stream over a cordierite monolith-supported Cu-Mn Hopcalite catalyst[J]. Applied Catalysis A:General,2012,427/428:73-78. doi: 10.1016/j.apcata.2012.03.035 [19] MARINOIU A, RACEANU M, COBZARU C, et al. Low temperature CO retention using hopcalite catalyst for fuel cell applications[J]. Reaction Kinetics, Mechanisms and Catalysis,2014,112(1):37-50. doi: 10.1007/s11144-014-0694-2 [20] LAMB A B, BRAY W C, FRAZER J C W. The removal of carbon monoxide from air[J]. Journal of Industrial & Engineering Chemistry,1920,12(3):213-221. [21] DEY S, DHAL G C, MOHAN D, et al. Application of hopcalite catalyst for controlling carbon monoxide emission at cold-start emission conditions[J]. Journal of Traffic and Transportation Engineering (English Edition),2019,6(5):419-440. doi: 10.1016/j.jtte.2019.06.002 [22] DEY S, DHAL G C. Synthesis of Hopcalite catalysts by various methods for improved catalytic conversion of carbon monoxide[J]. Materials Science for Energy Technologies,2020,3:377-389. doi: 10.1016/j.mset.2020.02.005 [23] DEY S, DHAL G C. Deactivation and regeneration of hopcalite catalyst for carbon monoxide oxidation: a review[J]. Materials Today Chemistry,2019,14:100180. doi: 10.1016/j.mtchem.2019.07.002 [24] SOLSONA B, GARCIA T, AGOURAM S, et al. The effect of gold addition on the catalytic performance of copper manganese oxide catalysts for the total oxidation of propane[J]. Applied Catalysis B:Environmental,2011,101(3/4):388-396. [25] SUN M H, HUANG S Z, CHEN L H, et al. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine[J]. Chemical Society Reviews,2016,45(12):3479-3563. doi: 10.1039/C6CS00135A [26] AL-FATESH A S, IBRAHIM A A, ALSHAREKH A M, et al. Iron catalyst for decomposition of methane: influence of Al/Si ratio support[J]. Egyptian Journal of Petroleum,2018,27(4):1221-1225. doi: 10.1016/j.ejpe.2018.05.004 [27] ZHANG S, GUO Y Y, LI X Y, et al. The double peaks and symmetric path phenomena in the catalytic activity of Pd/Al2O3-TiO2 catalysts with different TiO2 contents[J]. Journal of Solid State Chemistry,2018,262:335-342. doi: 10.1016/j.jssc.2018.03.036 [28] TANG W X, LI W H, LI D Y, et al. Synergistic effects in porous Mn-Co mixed oxide nanorods enhance catalytic deep oxidation of benzene[J]. Catalysis Letters,2014,144(11):1900-1910. doi: 10.1007/s10562-014-1340-3 [29] LIU C X, GONG L, DAI R Y, et al. Mesoporous Mn promoted Co3O4 oxides as an efficient and stable catalyst for low temperature oxidation of CO[J]. Solid State Sciences,2017,71:69-74. doi: 10.1016/j.solidstatesciences.2017.07.006 [30] MORALES M R, BARBERO B P, CADÚS L E. Total oxidation of ethanol and propane over Mn-Cu mixed oxide catalysts[J]. Applied Catalysis B:Environmental,2006,67(3/4):229-236. [31] WASKOWSKA A, GERWARD L, STAUN OLSEN J, et al. CuMn2O4: properties and the high-pressure induced Jahn-Teller phase transition[J]. Journal of Physics:Condensed Matter,2001,13(11):2549-2562. doi: 10.1088/0953-8984/13/11/311 [32] VEPŘEK S, COCKE D L, KEHL S, et al. Mechanism of the deactivation of Hopcalite catalysts studied by XPS, ISS, and other techniques[J]. Journal of Catalysis,1986,100(1):250-263. doi: 10.1016/0021-9517(86)90090-4 [33] CHEN H, TONG X L, LI Y D. Mesoporous Cu-Mn Hopcalite catalyst and its performance in low temperature ethylene combustion in a carbon dioxide stream[J]. Applied Catalysis A: General,2009,370(1/2):59-65. [34] LI Y R, PENG H G, XU X L, et al. Facile preparation of mesoporous Cu-Sn solid solutions as active catalysts for CO oxidation[J]. RSC Advances,2015,5(33):25755-25764. doi: 10.1039/C5RA00635J [35] ARENA F, TORRE T, RAIMONDO C, et al. Structure and redox properties of bulk and supported manganese oxide catalysts[J]. Physical Chemistry Chemical Physics,2001,3(10):1911-1917. doi: 10.1039/b100091h [36] 朱学诚. 适用于过滤催化复合材料的锰基催化剂低温氧化挥发性有机物的机理研究[D]. 杭州: 浙江大学, 2019. [37] YE Z, GIRAUDON J M, NUNS N, et al. Influence of the preparation method on the activity of copper-manganese oxides for toluene total oxidation[J]. Applied Catalysis B: Environmental,2018,233:154-166. doi: 10.1016/j.apcatb.2017.06.072 [38] WU Y S, LU Y, SONG C J, et al. A novel redox-precipitation method for the preparation of α-MnO2 with a high surface Mn4+ concentration and its activity toward complete catalytic oxidation of o-xylene[J]. Catalysis Today,2013,201:32-39. doi: 10.1016/j.cattod.2012.04.032 [39] DU X S, GAO X, QU R Y, et al. The influence of alkali metals on the Ce-Ti mixed oxide catalyst for the selective catalytic reduction of NOx[J]. ChemCatChem,2012,4(12):2075-2081. doi: 10.1002/cctc.201200316 [40] DU X S, GAO X, CUI L W, et al. Investigation of the effect of Cu addition on the SO2-resistance of a CeTi oxide catalyst for selective catalytic reduction of NO with NH3[J]. Fuel,2012,92(1):49-55. doi: 10.1016/j.fuel.2011.08.014 [41] WU Y S, ZHANG Y X, LIU M, et al. Complete catalytic oxidation of o-xylene over Mn-Ce oxides prepared using a redox-precipitation method[J]. Catalysis Today,2010,153(3/4):170-175. [42] 王幸宜. 催化剂表征[M]. 上海: 华东理工大学出版社, 2008: 204-209. [43] DU H W, WANG Y, WAN T, et al. Highly efficient and selective Cu/MnOx catalysts for carbon dioxide reduction[J]. ACS Applied Energy Materials,2018,1(7):3035-3041. doi: 10.1021/acsaem.8b00548 [44] LI J H, LIANG X, XU S C, et al. Catalytic performance of manganese cobalt oxides on methane combustion at low temperature[J]. Applied Catalysis B:Environmental,2009,90(1/2):307-312. [45] KALIYA PERUMAL VEERAPANDIAN S, GIRAUDON J M, de GEYTER N, et al. Regeneration of Hopcalite used for the adsorption plasma catalytic removal of toluene by non-thermal plasma[J]. Journal of Hazardous Materials,2021,402:123877. doi: 10.1016/j.jhazmat.2020.123877 [46] WU Z B, JIN R B, LIU Y, et al. Ceria modified MnOx/TiO2 as a superior catalyst for NO reduction with NH3 at low-temperature[J]. Catalysis Communications,2008,9(13):2217-2220. doi: 10.1016/j.catcom.2008.05.001 [47] BIEMELT T, WEGNER K, TEICHERT J, et al. Hopcalite nanoparticle catalysts with high water vapour stability for catalytic oxidation of carbon monoxide[J]. Applied Catalysis B:Environmental,2016,184:208-215. □ doi: 10.1016/j.apcatb.2015.11.008 -





 下载:
下载: