α-Fe2O3 catalytic ozonation coupled with ceramic membrane for phenol wastewater treatment
-
摘要:
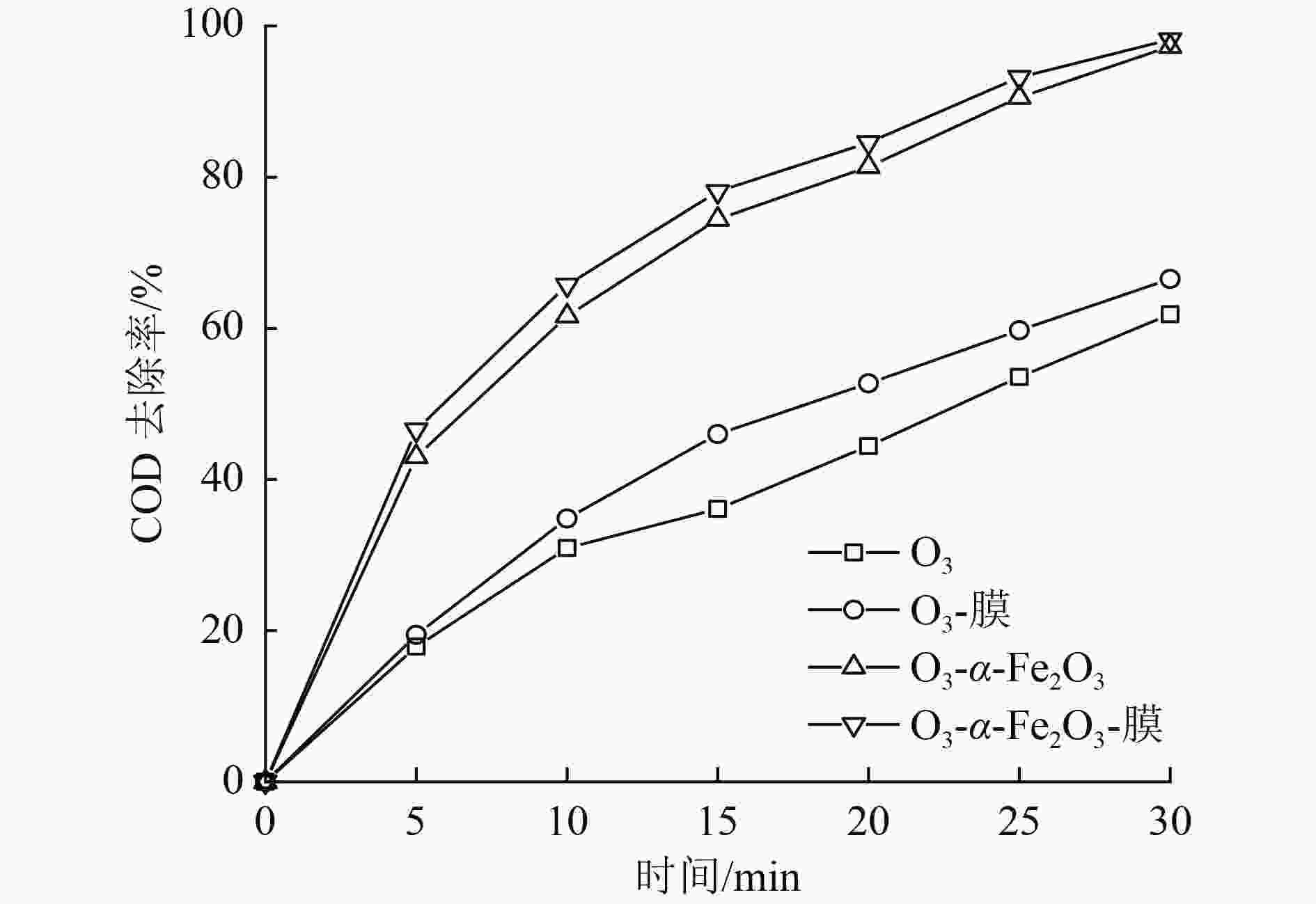
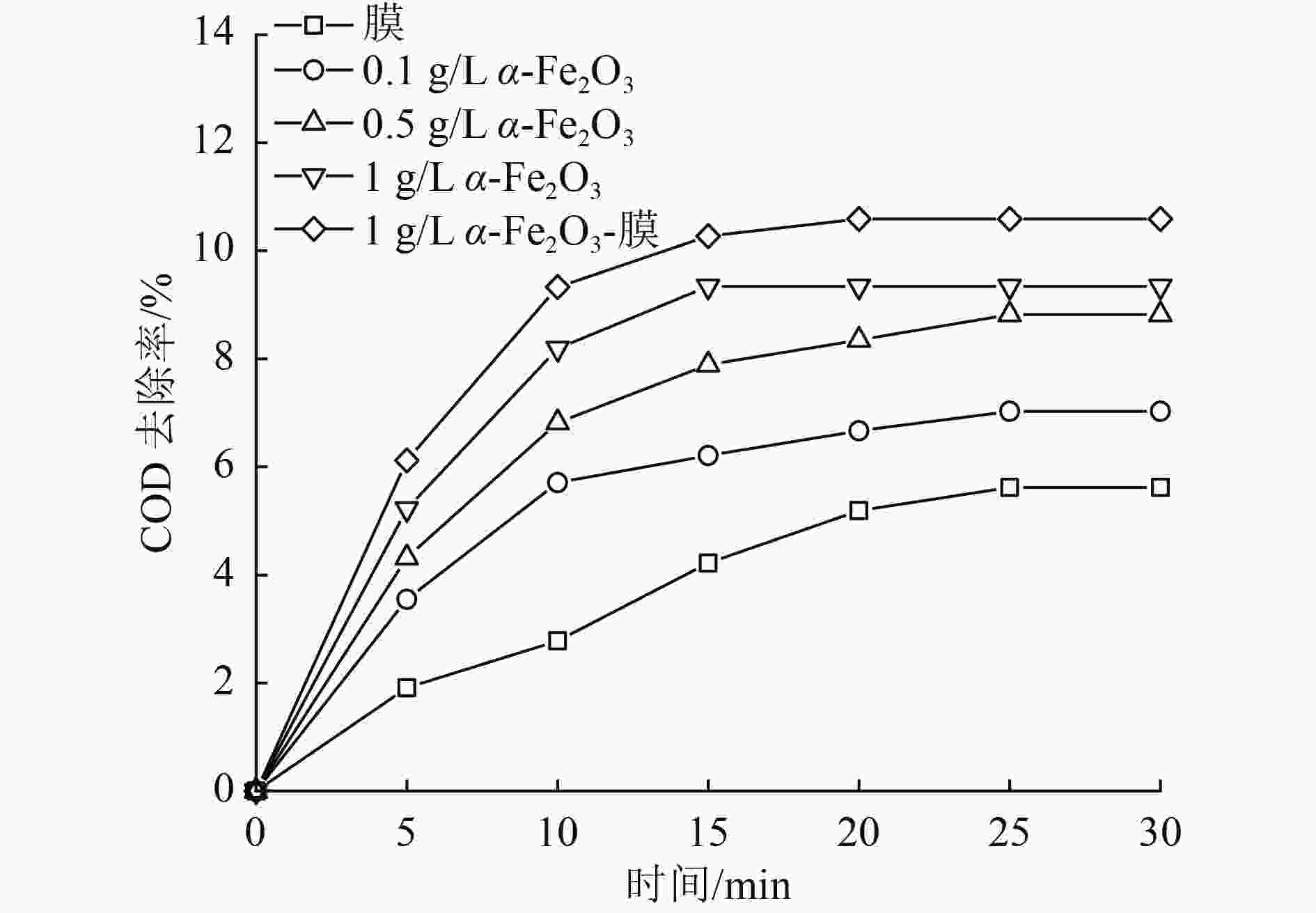
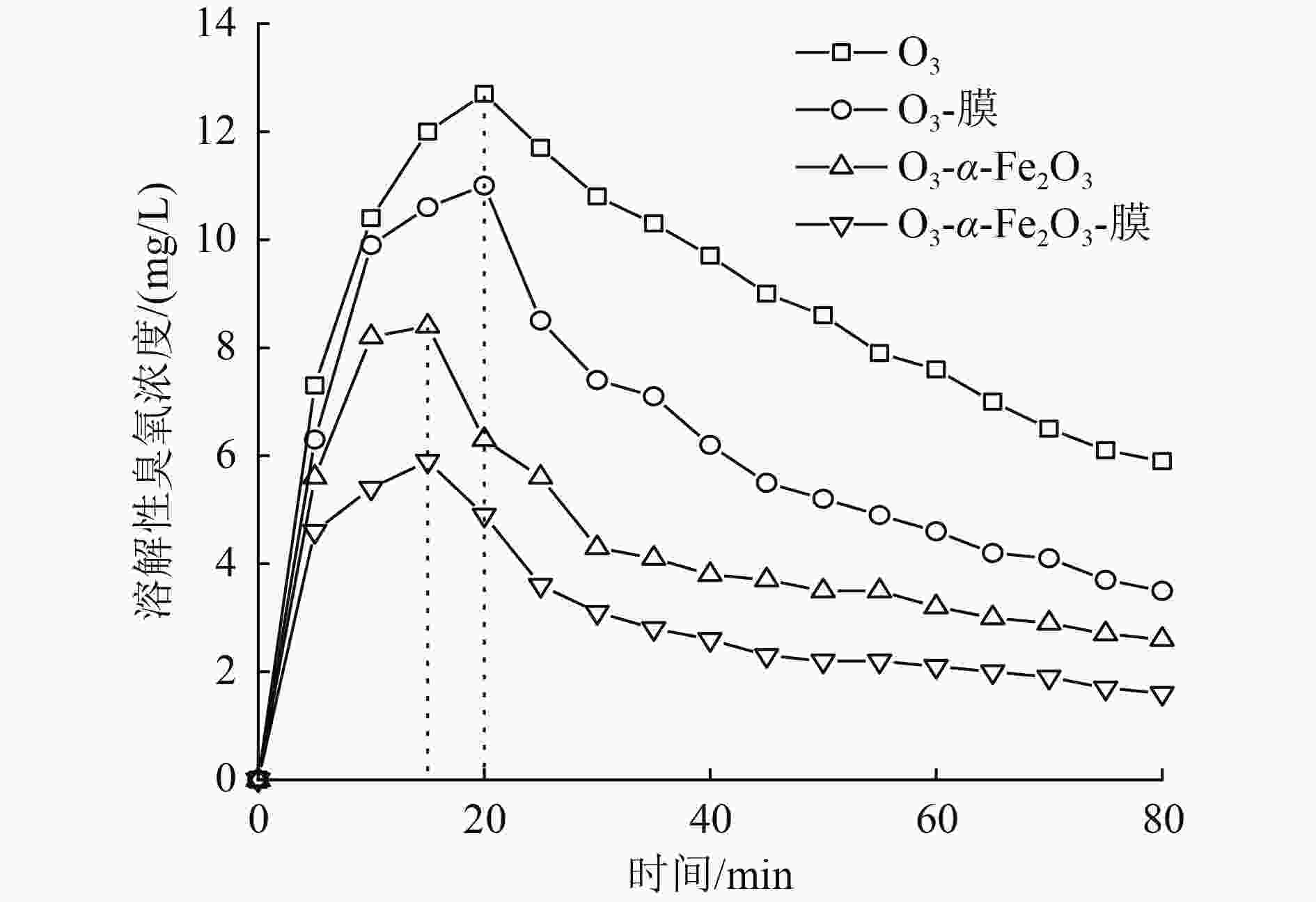
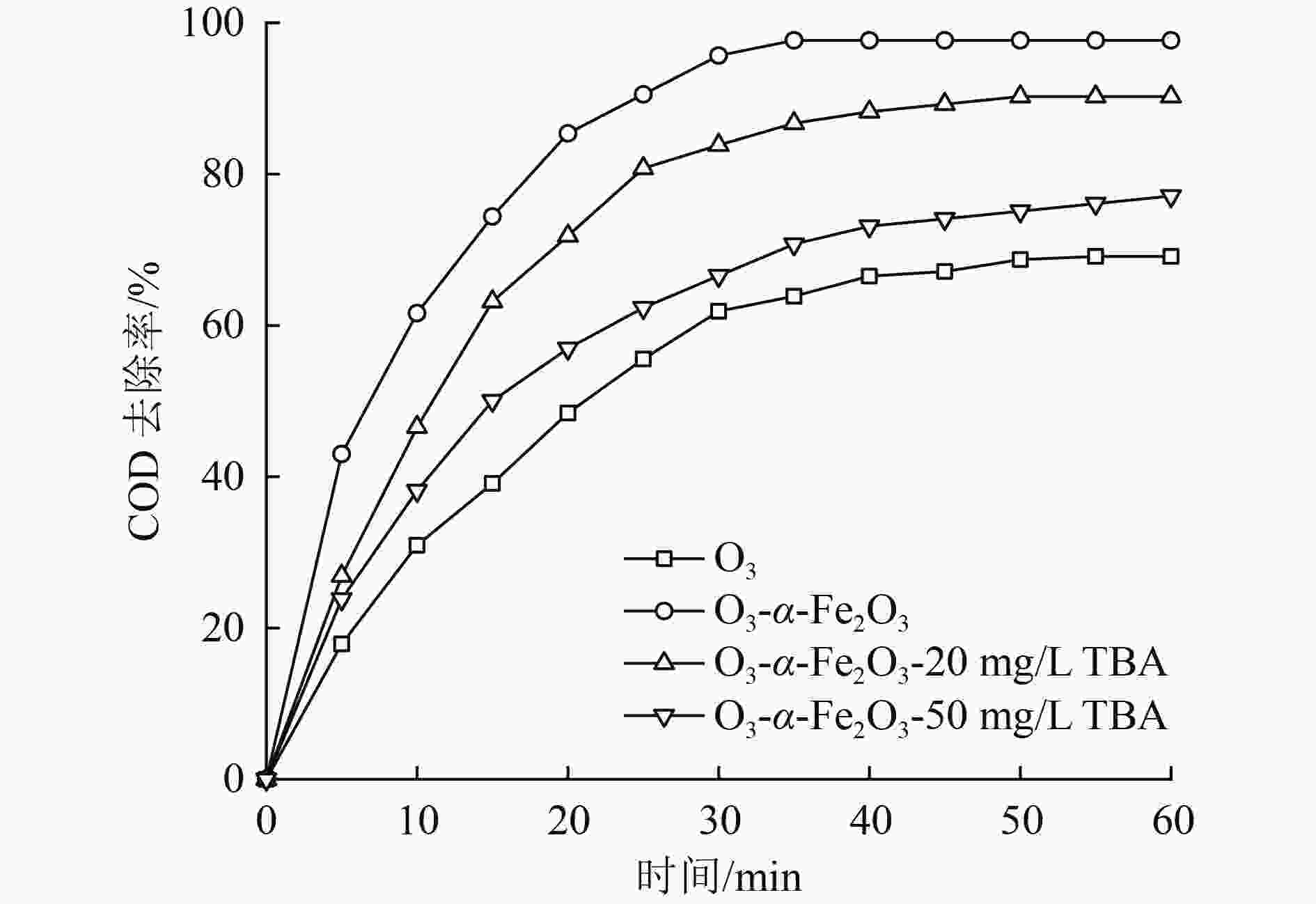
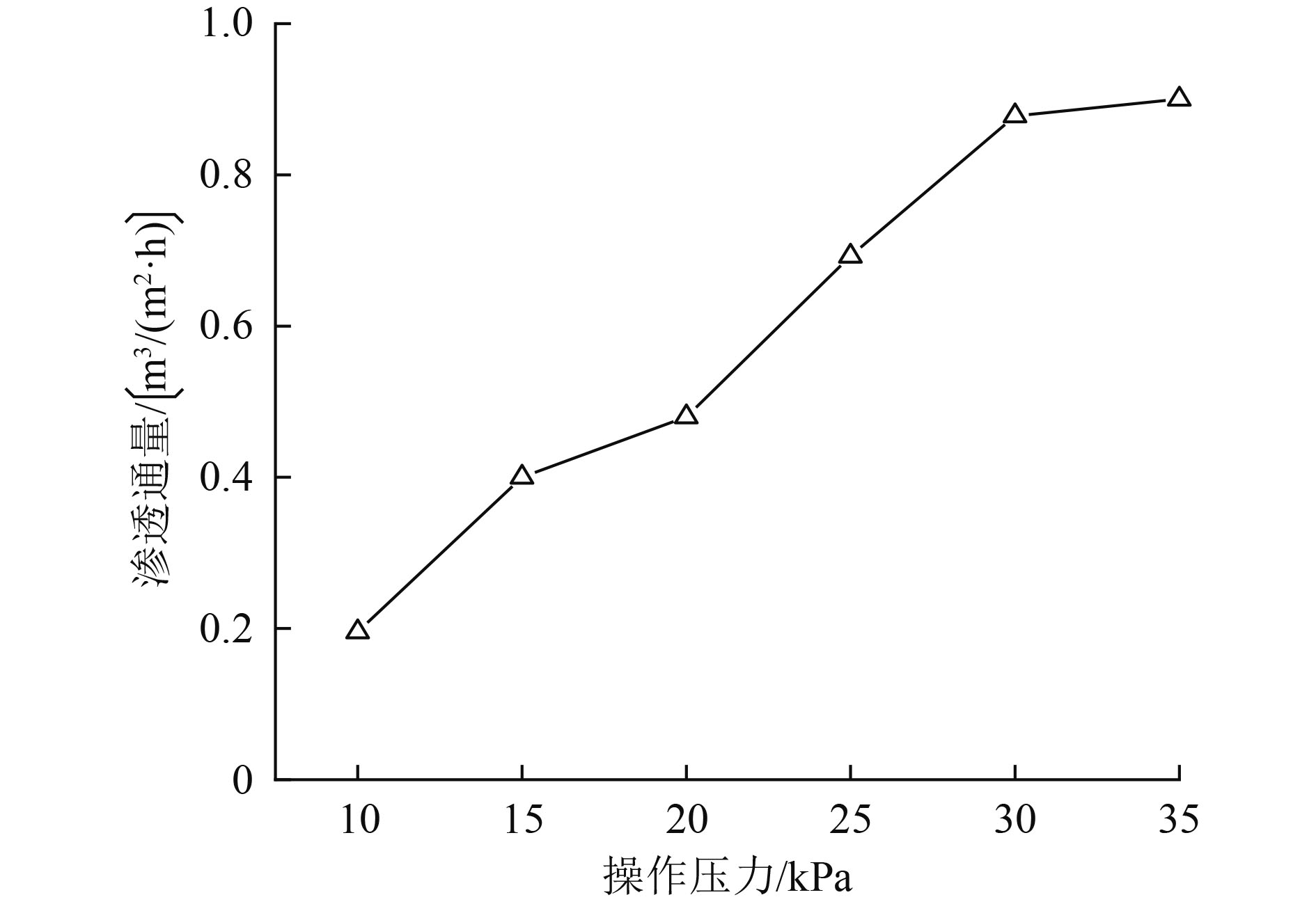
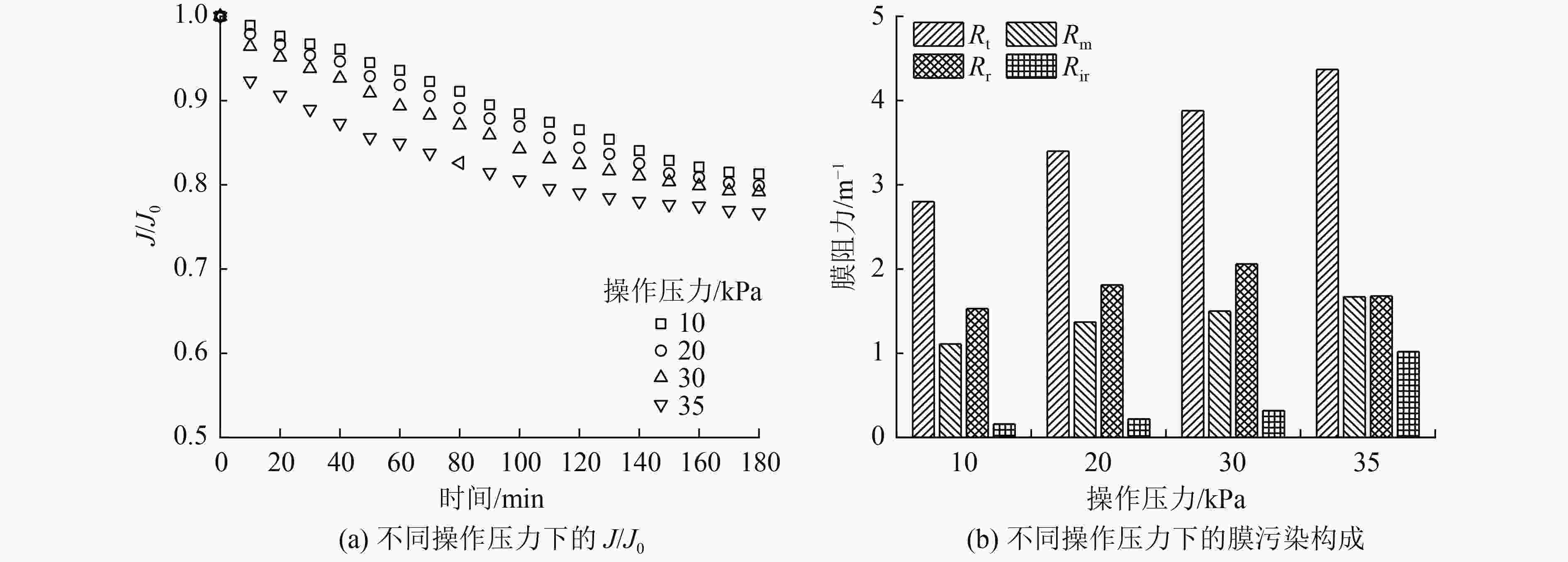
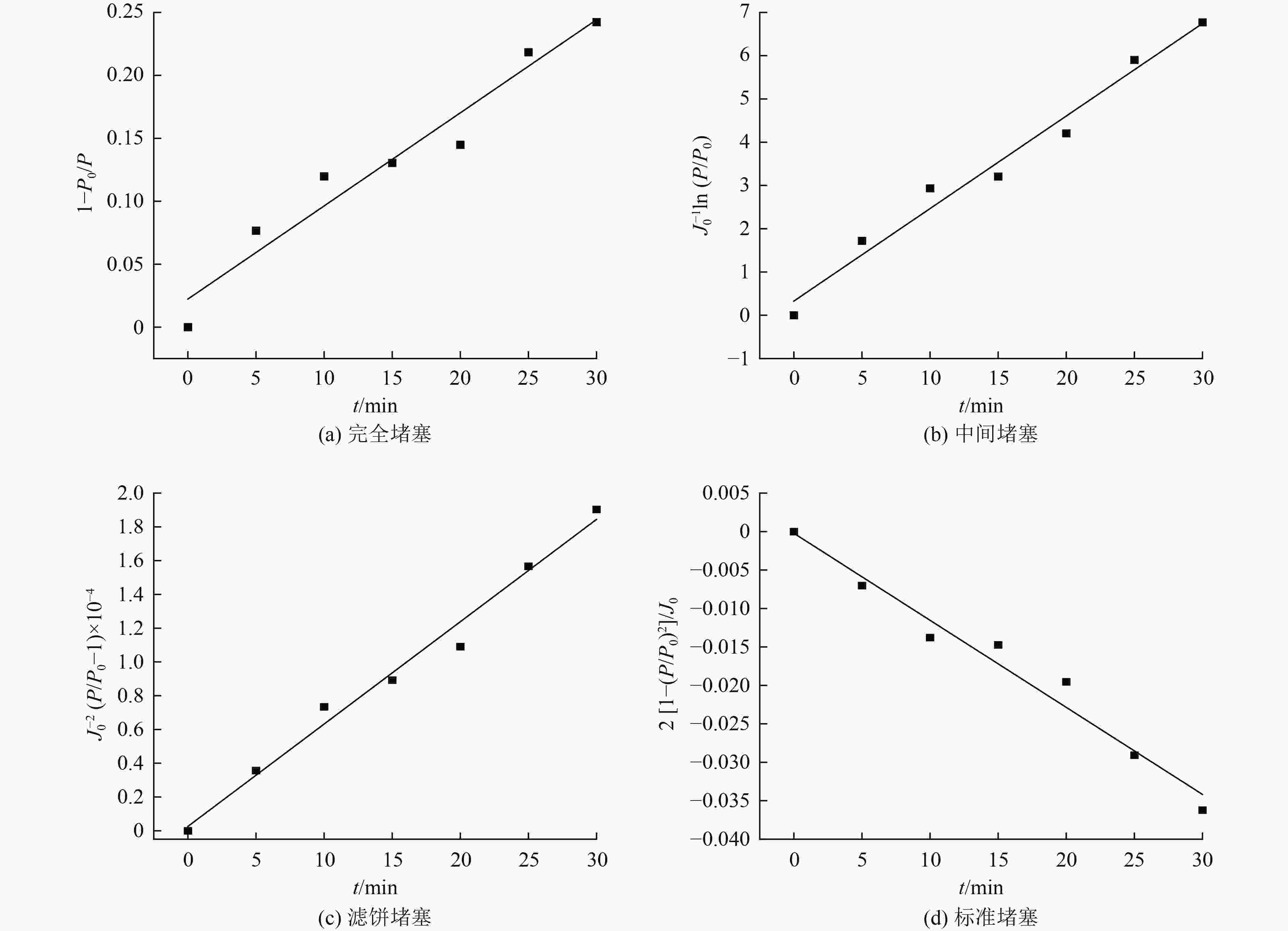
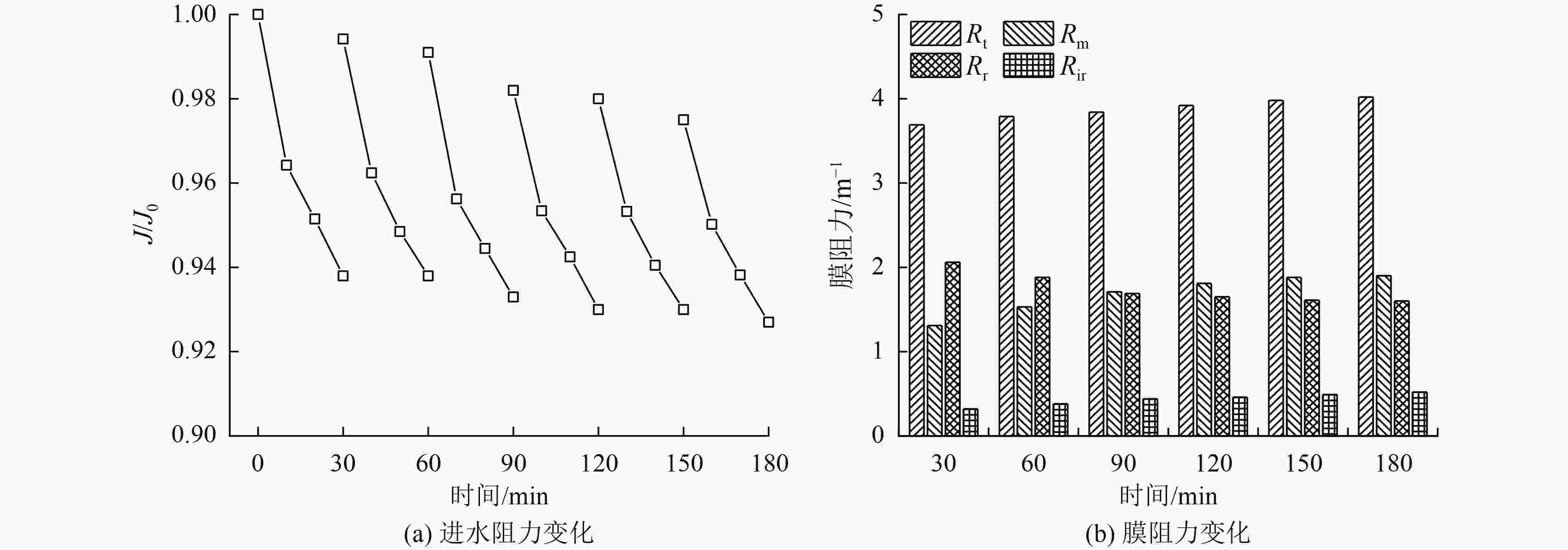
催化臭氧氧化是处理含酚废水的有效手段,为研究α-Fe2O3催化氧化含酚废水的降解效能同时有效回收催化剂,采用微米级α-Fe2O3催化臭氧氧化苯酚模拟废水,并耦合陶瓷膜对分散在反应体系的催化剂进行截留、回收,实现工艺的连续运行。结果表明:在间歇运行条件下,催化氧化反应30 min时废水COD去除率达到97%以上,高COD去除率的主要原因是α-Fe2O3对臭氧具有较强的催化活性,在催化氧化过程中产生了强氧化性产物·OH;在恒压条件下,通过膜污染模型拟合和串联阻力模型进行验证,Rr占总阻力的50%以上,但当操作压力超过30 kPa,一部分可逆污染向不可逆污染逐渐转化,Rir显著增加;通过动力学拟合探究膜污染形成机制,运行过程中陶瓷膜污染模型为中间堵塞或滤饼堵塞,膜污染主要发生在膜表面,膜可以对α-Fe2O3进行有效拦截并通过反冲洗恢复通量;连续进水6个周期运行过程中,模拟废水COD去除率保持在85%以上,陶瓷膜不可逆阻力控制在总阻力的13%以下,反应体系保持了稳定运行。
Abstract:Catalytic ozonation is an effective method for the treatment of phenolic wastewater. In order to study the degradation efficiency of phenolic wastewater by α-Fe2O3 catalytic oxidation and effectively recover the catalyst, micron-sized α-Fe2O3 catalytic ozonation was applied to the simulated phenol wastewater, and the catalyst dispersed in the reaction system was intercepted and recovered by the ceramic membrane to realize the continuous operation of the process. The results showed that: Under the condition of intermittent operation, COD removal rate of wastewater reached more than 97% after catalytic oxidation reaction for 30 min. The main reason for the high COD removal rate was that α-Fe2O3 had strong catalytic activity for ozone and strong oxidizing product ·OH was produced during catalytic oxidation.Under the condition of constant pressure, Rr accounted for more than 50% of the total resistance, which was verified by membrane fouling model fitting and series resistance model. However, when the operating pressure exceeded 30 kPa, some reversible fouling gradually transformed into irreversible fouling, and Rir increased significantly. The formation mechanism of membrane fouling was explored by kinetic fitting. The ceramic membrane fouling model during operation was intermediate blockage or filter cake blockage. Membrane fouling mainly occurred on the membrane surface. The membrane could effectively intercept α-Fe2O3 and recover the flux through backwashing. During the six-cycle operation of continuous influent, COD removal rate of simulated wastewater remained above 85%, the irreversible resistance of ceramic membrane was controlled below 13% of the total resistance, and the reaction system maintained stable operation.
-
Key words:
- α-Fe2O3 /
- catalytic ozonation /
- phenol /
- ceramic membrane /
- membrane fouling /
- membrane resistance
-
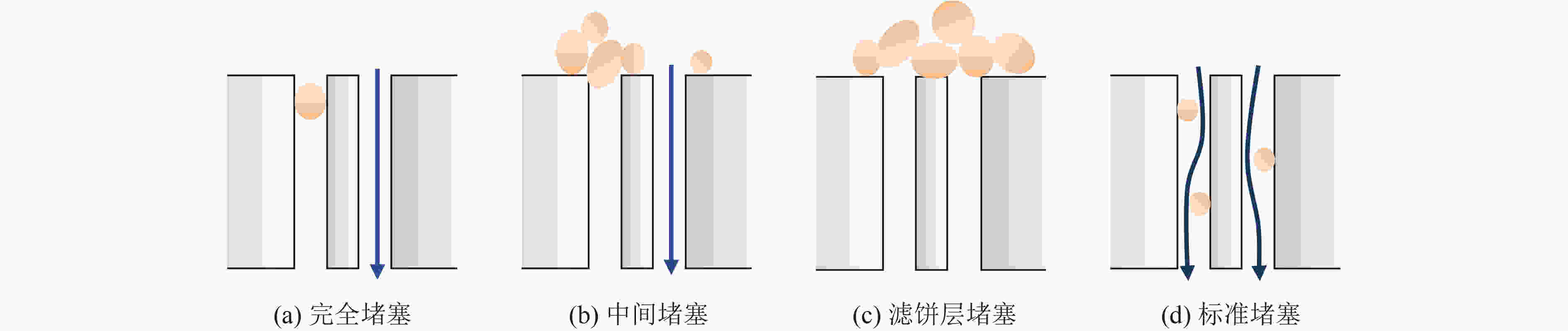
表 1 膜堵塞模型公式
Table 1. Formula of membrane blocking model
污染模型 模型公式 完全堵塞 $\dfrac{P}{ { {P_0} } } = \dfrac{1}{ {1 - {k_{\rm{b}}}t} }$ 中间堵塞 $\dfrac{P}{ { {P_0} } } = \exp ({k_{\rm{i}}}{J_0}t)$ 滤饼堵塞 $\dfrac{P}{ { {P_0} } } = 1 + {k_{\rm{c}}}{J_0}^2t$ 标准堵塞 $\dfrac{P}{ { {P_0} } } = {\left(1 - \dfrac{ { {k_{\rm{s}}}{J_0}t} }{2}\right)^{ - 2} }$ 注:P和P0当前状态的跨膜压差(TMP),kPa;$ {k_{\rm{b}}} $、${k_{\rm{i}}}$、$ {k_{\rm{c}}} $及$ {k_{\rm{s}}} $为污染模型的拟合参数。 表 2 催化氧化准一级动力学参数
Table 2. Pseudo-first-order kinetics parameters of catalytic oxidation
反应条件 动力学参数 k/s−1 R2 S O3 −0.110 0.986 0.993 O3-膜 −0.118 0.987 0.993 O3-α-Fe2O3 −0.304 0.990 0.995 O3-α-Fe2O3-膜 −0.356 0.994 0.997 表 3 连续运行过程COD去除率变化
Table 3. Change of COD removal rate during continuous operation
时间/min 30 60 90 120 150 180 COD去除率/% 86.91 86.25 85.28 86.33 86.13 86.01 -
[1] WU J F, SU T M, JIANG Y X, et al. In situ DRIFTS study of O3 adsorption on CaO, γ-Al2O3, CuO, α-Fe2O3 and ZnO at room temperature for the catalytic ozonation of cinnamaldehyde[J]. Applied Surface Science,2017,412:290-305. doi: 10.1016/j.apsusc.2017.03.237 [2] DENG S H, JOTHINATHAN L, CAI Q Q, et al. FeOx@GAC catalyzed microbubble ozonation coupled with biological process for industrial phenolic wastewater treatment: catalytic performance, biological process screening and microbial characteristics[J]. Water Research,2021,190:116687. doi: 10.1016/j.watres.2020.116687 [3] LI X F, CHEN W Y, MA L M, et al. Industrial wastewater advanced treatment via catalytic ozonation with an Fe-based catalyst[J]. Chemosphere,2018,195:336-343. doi: 10.1016/j.chemosphere.2017.12.080 [4] MECHA A C, CHOLLOM M N. Photocatalytic ozonation of wastewater: a review[J]. Environmental Chemistry Letters,2020,18(5):1491-1507. doi: 10.1007/s10311-020-01020-x [5] EINAGA H, MAEDA N, NAGAI Y. Comparison of catalytic properties of supported metal oxides for benzene oxidation using ozone[J]. Catalysis Science & Technology,2015,5(6):3147-3158. [6] 任越中, 张嘉雯, 魏健, 等.铈负载改性天然沸石催化臭氧氧化水中青霉素G[J]. 环境工程技术学报,2019,9(1):28-35. doi: 10.3969/j.issn.1674-991X.2019.01.005REN Y Z, ZHANG J W, WEI J, et al. Catalytic ozonation of penicillin G in aqueous phase using modified natural zeolite supported cerium[J]. Journal of Environmental Engineering Technology,2019,9(1):28-35. doi: 10.3969/j.issn.1674-991X.2019.01.005 [7] 付丽亚, 吴昌永, 周鉴, 等.3种一体式臭氧-BAF工艺对石化废水生化出水有机物去除特性比较研究[J]. 环境工程技术学报,2021,11(1):135-143. doi: 10.12153/j.issn.1674-991X.20200061FU L Y, WU C Y, ZHOU J, et al. Comparison study of organics removal characteristics by three kinds of integrated ozone-BAF processes treating biochemical effluent of petrochemical wastewater[J]. Journal of Environmental Engineering Technology,2021,11(1):135-143. doi: 10.12153/j.issn.1674-991X.20200061 [8] 李亚男, 谭煜, 吴昌永, 等.臭氧催化氧化在石化废水深度处理应用中的若干问题[J]. 环境工程技术学报,2019,9(3):275-281. doi: 10.12153/j.issn.1674-991X.2019.02.280LI Y N, TAN Y, WU C Y, et al. Application and problems of catalytic ozonation in advanced treatment of petrochemical wastewater[J]. Journal of Environmental Engineering Technology,2019,9(3):275-281. doi: 10.12153/j.issn.1674-991X.2019.02.280 [9] YANG W W, LU Z, VOGLER B, et al. Enhancement of copper catalyst stability for catalytic ozonation in water treatment using ALD overcoating[J]. ACS Applied Materials & Interfaces,2018,10(50):43323-43326. [10] LIANG X S, WANG L S, WEN T C, et al. Mesoporous poorly crystalline α-Fe2O3 with abundant oxygen vacancies and acid sites for ozone decomposition[J]. Science of the Total Environment,2022,804:150161. doi: 10.1016/j.scitotenv.2021.150161 [11] TAN X Q, WAN Y F, HUANG Y J, et al. Three-dimensional MnO2 porous hollow microspheres for enhanced activity as ozonation catalysts in degradation of bisphenol A[J]. Journal of Hazardous Materials,2017,321:162-172. doi: 10.1016/j.jhazmat.2016.09.013 [12] WANG B, XIONG X, REN H Y, et al. Preparation of MgO nanocrystals and catalytic mechanism on phenol ozonation[J]. RSC Advances,2017,7(69):43464-43473. doi: 10.1039/C7RA07553G [13] LIN F W, WANG Z H, MA Q, et al. Catalytic deep oxidation of NO by ozone over MnOx loaded spherical alumina catalyst[J]. Applied Catalysis B:Environmental,2016,198:100-111. doi: 10.1016/j.apcatb.2016.05.058 [14] HOU S, JIA S Y, JIA J J, et al. Fe3O4 nanoparticles loading on cow dung based activated carbon as an efficient catalyst for catalytic microbubble ozonation of biologically pretreated coal gasification wastewater[J]. Journal of Environmental Management,2020,267:110615. doi: 10.1016/j.jenvman.2020.110615 [15] EINAGA H, OGATA A. Benzene oxidation with ozone over supported manganese oxide catalysts: effect of catalyst support and reaction conditions[J]. Journal of Hazardous Materials,2009,164(2/3):1236-1241. [16] RAYATI S, POURNASER N, NEJABAT F, et al. Aerobic oxidation of cyclohexene over Mn-porphyrin based nanocatalyst: supported vs unsupported catalyst[J]. Inorganic Chemistry Communications,2019,107:107447. doi: 10.1016/j.inoche.2019.107447 [17] XIONG W, CHEN N, FENG C P, et al. Ozonation catalyzed by iron- and/or manganese-supported granular activated carbons for the treatment of phenol[J]. Environmental Science and Pollution Research International,2019,26(20):21022-21033. doi: 10.1007/s11356-019-05304-w [18] DANG T T, DO V M, TRINH V T. Nano-catalysts in ozone-based advanced oxidation processes for wastewater treatment[J]. Current Pollution Reports,2020,6(3):217-229. doi: 10.1007/s40726-020-00147-3 [19] 陈天翼, 李根, 王卓, 等.粉末活性炭-陶瓷膜臭氧催化氧化深度处理煤气化废水研究[J]. 水处理技术,2018,44(2):80-83. doi: 10.16796/j.cnki.1000-3770.2018.02.019CHEN T Y, LI G, WANG Z, et al. Advanced treatment of coal gasification wastewater by powdered activated carbon-ceramic membrane catalytic ozonation[J]. Technology of Water Treatment,2018,44(2):80-83. doi: 10.16796/j.cnki.1000-3770.2018.02.019 [20] ZHONG Z X, LI D Y, LIU X, et al. The fouling mechanism of ceramic membranes used for recovering TS-1 catalysts[J]. Chinese Journal of Chemical Engineering,2009,17(1):53-57. doi: 10.1016/S1004-9541(09)60032-X [21] WU Z J, HOU Y Q, LI X M, et al. Pilot study on catalyzed oxidation-ceramic membrane-high pressure reverse osmosis for desulfurization wastewater recovery[J]. IOP Conference Series:Earth and Environmental Science,2021,668(1):012033. doi: 10.1088/1755-1315/668/1/012033 [22] ZHANG J L, YU H T, QUAN X, et al. Ceramic membrane separation coupled with catalytic ozonation for tertiary treatment of dyestuff wastewater in a pilot-scale study[J]. Chemical Engineering Journal,2016,301:19-26. doi: 10.1016/j.cej.2016.04.148 [23] di LUCA C, INCHAURRONDO N, MARCÉ M, et al. On disclosing the role of mesoporous alumina in the ozonation of sulfamethoxazole: adsorption vs. catalysis[J]. Chemical Engineering Journal,2021,412:128579. doi: 10.1016/j.cej.2021.128579 [24] LUO X, SU T M, XIE X L, et al. The adsorption of ozone on the solid catalyst surface and the catalytic reaction mechanism for organic components[J]. ChemistrySelect,2020,5(48):15092-15116. doi: 10.1002/slct.202003805 [25] RUIZ J A, RODRÍGUEZ J L, POZNYAK T, et al. Catalytic effect of γ-Al(OH)3, α-FeOOH, and α-Fe2O3 on the ozonation-based decomposition of diethyl phthalate adsorbed on sand and soil[J]. Environmental Science and Pollution Research,2021,28(1):974-981. doi: 10.1007/s11356-020-10522-8 [26] MEHANDJIEV D, NAIDENOV A. Ozone decomposition on α-Fe2O3 catalyst[J]. Ozone:Science & Engineering,1992,14(4):277-282. [27] LI Y, WU L C, WANG Y, et al. γ-Al2O3 doped with cerium to enhance electron transfer in catalytic ozonation of phenol[J]. Journal of Water Process Engineering,2020,36:101313. doi: 10.1016/j.jwpe.2020.101313 [28] WITKOWSKI B, JURDANA S, GIERCZAK T. Limononic acid oxidation by hydroxyl radicals and ozone in the aqueous phase[J]. Environmental Science & Technology,2018,52(6):3402-3411. [29] MIKULÁŠEK P, DOLEČEK P, ŠMÍDOVÁ D, et al. Crossflow microfiltration of mineral dispersions using ceramic membranes[J]. Desalination,2004,163(1/2/3):333-343. [30] OLIVEIRA NETO G L, OLIVEIRA N G N, DELGADO J M P Q, et al. A new design of tubular ceramic membrane module for oily water treatment: multiphase flow behavior and performance evaluation[J]. Membranes,2020,10(12):403. doi: 10.3390/membranes10120403 [31] DERISZADEH A, HUSEIN M M, HARDING T G. Produced water treatment by micellar-enhanced ultrafiltration[J]. Environmental Science & Technology,2010,44(5):1767-1772. [32] XU J, CHANG C Y, GAO C J. Performance of a ceramic ultrafiltration membrane system in pretreatment to seawater desalination[J]. Separation and Purification Technology,2010,75(2):165-173. doi: 10.1016/j.seppur.2010.07.020 [33] WANG X L, LI Y L, YU H T, et al. High-flux robust ceramic membranes functionally decorated with nano-catalyst for emerging micro-pollutant removal from water[J]. Journal of Membrane Science,2020,611:118281. doi: 10.1016/j.memsci.2020.118281 [34] YAN L Q, BING J S, WU H C. The behavior of ozone on different iron oxides surface sites in water[J]. Scientific Reports,2019,9:14752. ⊗ doi: 10.1038/s41598-019-50910-w -




 下载:
下载: