Synthesis of a new water treatment agent and its selective removal of anionic pollutants
-
摘要:
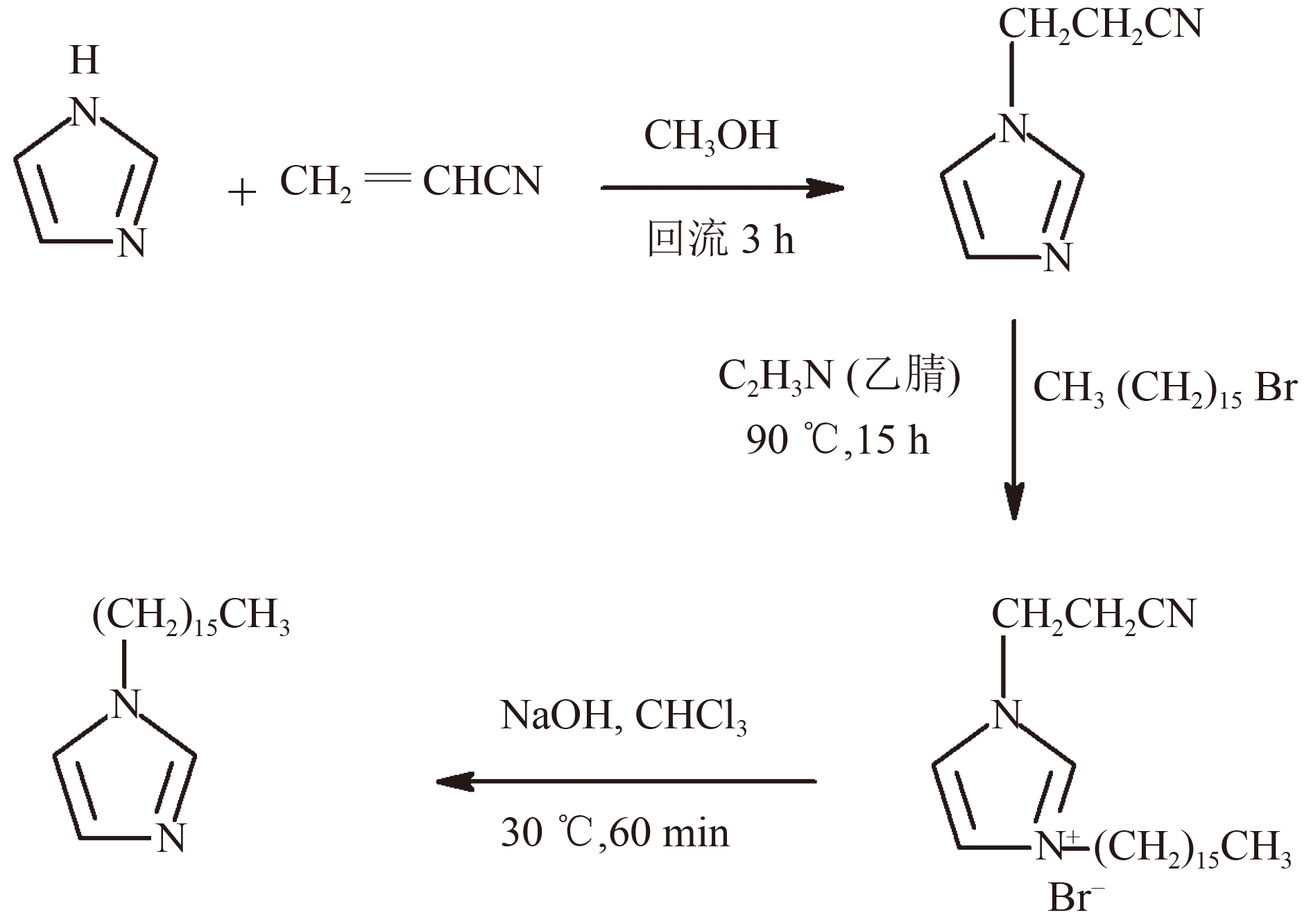
通过键合法合成了一种新型水处理剂—氯甲基聚苯乙烯树脂固载的功能化离子液体〔PS-CH2-(CH2)16Im〕,选取Cr(Ⅵ)和甲基橙(MO)为模型阴离子污染物,采用静态与动态柱试验对其选择性去除水中阴离子污染物的性能进行系统研究。结果表明,PS-CH2-(CH2)16Im对阴离子污染物选择性吸附性能良好,30 min可达吸附平衡,对Cr(Ⅵ)与MO的静态最大吸附量分别为90.9和62.8 mg/g,可有效去除低至0.004 mg/L的Cr(Ⅵ)。PS-CH2-(CH2)16Im易于再生,5次重复使用后吸附性能无显著降低。通过测定缔合常数,明确PS-CH2-(CH2)16Im对阴离子污染物的吸附机理为离子缔合作用。
Abstract:A novel water treatment agent, chloromethyl polystyrene resin supported task-specific ionic liquid(PS-CH2-(CH2)16Im) was synthesized by a bonding method. Cr(Ⅵ) and methyl orange (MO) were selected as model anionic pollutants. The performance of selective removal of anionic pollutants in water was systematically investigated by both static and dynamic column tests. The results showed that PS-CH2-(CH2)16Im had good selective adsorption for anionic pollutants. The adsorption equilibrium could be reached in 30 minutes, with static maximum adsorption capacity of 90.9 and 62.8 mg/g for Cr(Ⅵ) and MO, respectively, and also could effectively remove Cr(Ⅵ) at a concentration as low as 0.004 mg/L. PS-CH2-(CH2)16Im was easy to regenerate and recycle at least 5 times without obvious loss in adsorption performance. By measuring the association constant, it was known that the adsorption mechanism of PS-CH2-(CH2)16Im on anionic pollutants was ion association.
-
表 1 不同洗脱剂对Cr(Ⅵ)的洗脱效果
Table 1. Elution effect of different eluents on Cr(Ⅵ)
项目 HNO3浓度/(mol/L) NaOH浓度/(mol/L) 1 2 3 4 1 2 3 Cr(Ⅵ)洗脱率/% 95 87 85 83 85 89 91 表 2 不同洗脱剂对MO的洗脱效果
Table 2. Elution effect of different eluents on MO
项目 NH4Cl浓度/
(mol/L)NaCl浓度/
(mol/L)NaOH浓度/
(mol/L)HCl浓度/
(mol/L)1 1 1 1 2 3 4 6 12 MO洗脱
率/%1.43 0.90 0 100 89 80 79 66 0.08 -
[1] LUTY-BŁOCHO M, PODBORSKA A, MUSIELAK B, et al. The specialized twin-solution method for selective Pd(Ⅱ) ions determination and methyl orange removal[J]. Journal of Molecular Liquids,2021,340(15):116884. [2] LI Q H, DONG M, LI R, et al. Enhancement of Cr(Ⅵ) removal efficiency via adsorption/photocatalysis synergy using electrospun chitosan/g-C3N4/TiO2 nanofibers[J]. Carbohydrate Polymers,2021,253(1):117200. [3] YASEEN D A, SCHOLZ M, Textile dye wastewater characteristics and constituents of synthetic effluents: a critical review[J]. International Journal of Environmental Science and Technology, 2019, 16(2): 1193-1226. [4] 刘永德, 马琳, 孙旭镯, 等, 葫芦[6]脲对印染废水中染料曙红的吸附特性及影响因素[J]. 环境工程技术学报, 2020, 10(5): 837-844.LIU Y D, MA L, SUN X Z, et al. Adsorption characteristics and influencing factors of cucurbit[6]uril for dye eosin Y from printing and dying wastewater[J]. Journal of Environmental Engineering Technology, 2020, 10(5): 837-844. [5] 曲宏斌, 万东锦, 肖书虎, 等, 离子交换树脂对水中高氯酸根的吸附及其机理研究[J]. 环境工程技术学报, 2016, 6(4): 343-349.QU H B, WAN D J, XIAO S H, et al. Study on adsorption of perchlorate by anion exchange resins and its mechanisms[J]. Journal of Environmental Engineering Technology, 2016, 6(4): 343-349. [6] SAS O G, DOMÍNGUEZ I, GONZÁLEZ B, et al. , Liquid-liquid extraction of phenolic compounds from water using ionic liquids: literature review and new experimental data using [C2mim]FSI[J]. Journal of Environmental Management,2018,228:475-482. [7] 王孝平, 邢树礼.考马斯亮蓝法测定蛋白含量的研究[J]. 天津化工,2009(3):40-42. doi: 10.3969/j.issn.1008-1267.2009.03.016 [8] 刘长姣, 杨越越, 王妮, 等.茚三酮比色法测定黄秋葵氨基酸含量的不确定度评定[J]. 粮食与油脂,2019,32(9):92-95. doi: 10.3969/j.issn.1008-9578.2019.09.023 [9] 国家环境保护局. 水质六价铬的测定二苯碳酰二肼分光光度法: GB/T 7467—1987[S]. 北京: 中国环境科学出版社, 1987. [10] WAQAR D, ALAM M, HUSSEIN W. Conductometric study of interaction between dyes and surface active agents[J]. Pakistan Journal of Pharmaceutical Sciences,1995,8(1):39-44. [11] 王西新, 赵建玲, 杨浩, 等.N-烷基(C4~C16)咪唑的合成[J]. 化学试剂,2001,23(5):306-307. doi: 10.3969/j.issn.0258-3283.2001.05.023 [12] WANG X, WAN H, HAN M, et al. Removal of thiophene and its derivatives from model gasoline using polymer-supported metal chlorides ionic liquid moieties[J]. Industrial & Engineering Chemistry Research,2012,51(8):3418-3424. [13] LIN Y, WANG F, ZHANG Z, et al. Polymer-supported ionic liquids: synthesis, characterization and application in fuel desulfurization[J]. Fuel,2014,116:273-280. doi: 10.1016/j.fuel.2013.08.014 [14] COTORUELO L M, MARQUES M D, DIAZ F J, et al. Adsorbent ability of lignin-based activated carbons for the removal of p-nitrophenol from aqueous solutions[J]. Chemical Engineering Journal,2012,184:176-183. doi: 10.1016/j.cej.2012.01.026 [15] AN F, DU R, WANG X, et al. Adsorption of phenolic compounds from aqueous solution using salicylic acid type adsorbent[J]. Journal of Hazardous Materials,2012,201:74-81. [16] 环境保护部. 地表水环境质量标准: GB 3838—2002[S]. 北京: 中国环境科学出版社, 2002. [17] RAFATI A A, AZIZIAN S, CHAHARDOLI M. Conductometric studies of interaction between anionic dyes and cetylpyridinium bromide in water-alcohol mixed solvents[J]. Journal of Molecular Liquids,2008,137(1/2/3):80-87. ⊗ -





 下载:
下载: