Optimization and mechanism analysis of α-Fe2O3 catalytic ozone oxidation parameters for phenolic wastewater
-
摘要:
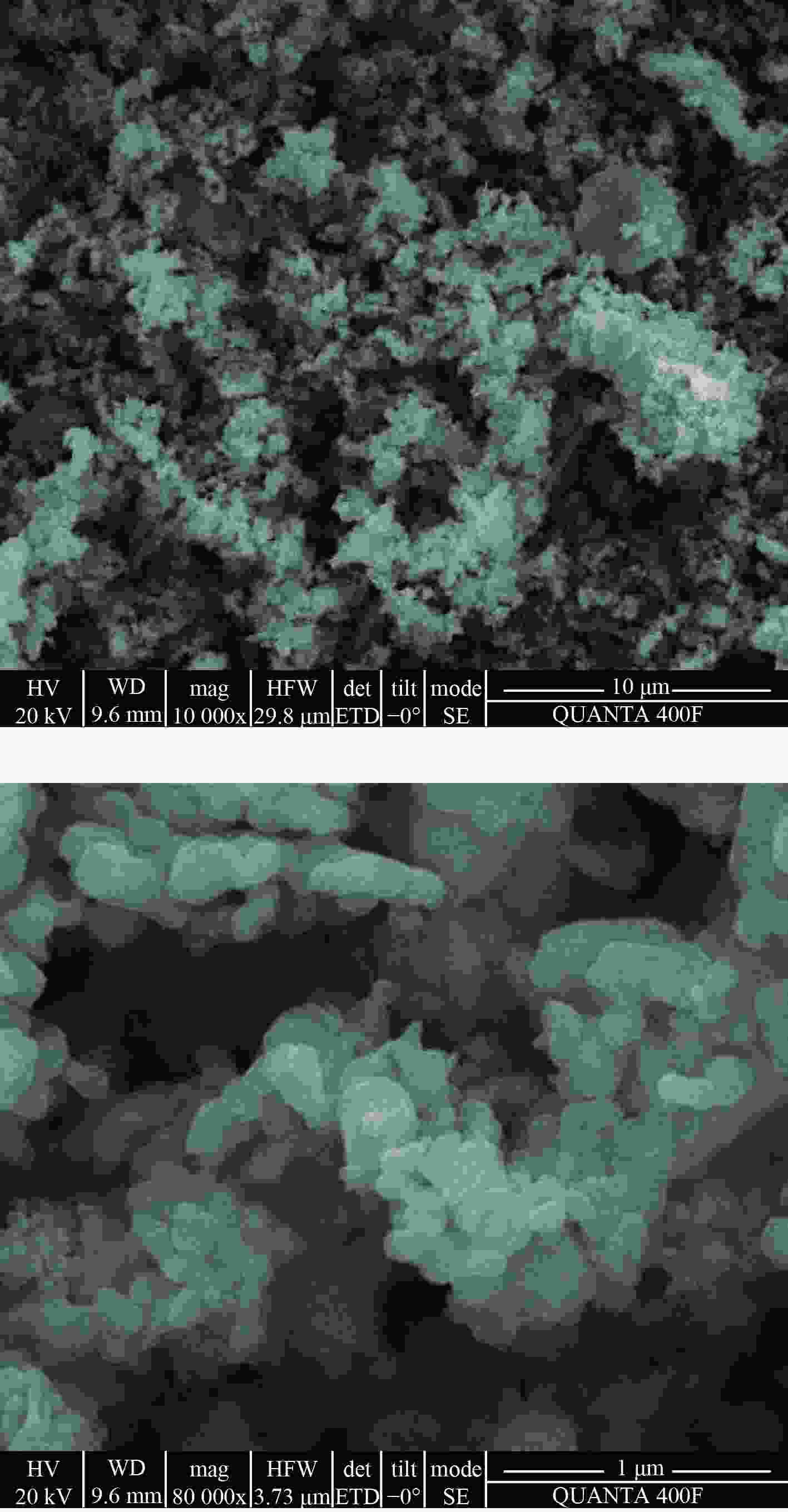
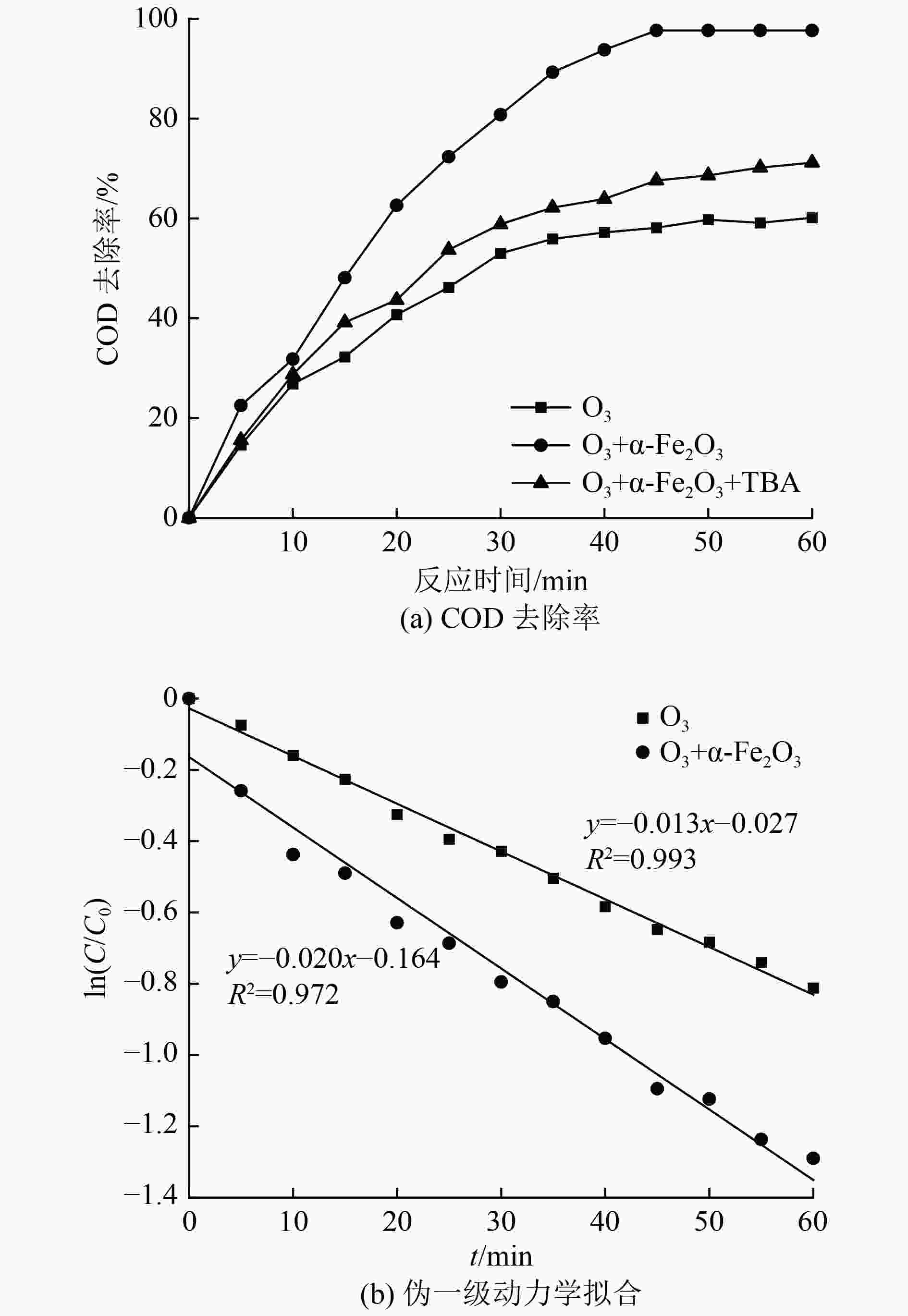
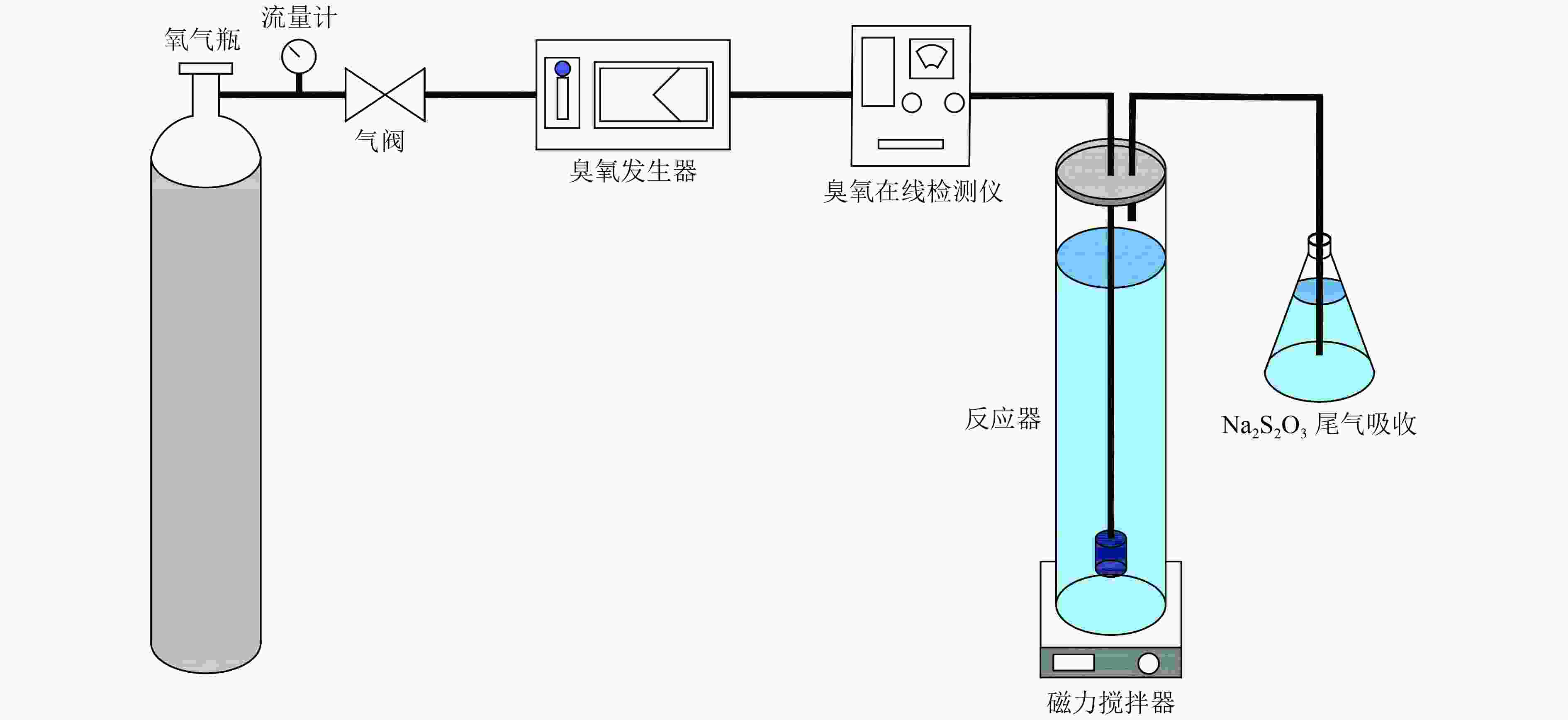
传统工艺对含酚废水的处理效果有限,催化臭氧氧化技术能够有效处理含酚废水。α-Fe2O3在试验中表现出了高臭氧催化活性,催化产生的·OH可对苯酚及中间产物进行无选择性矿化,显著增强了污染物去除效果和臭氧利用水平。为明确催化臭氧氧化过程主要影响因素并优化工艺参数,以苯酚模拟含酚废水,设计了L16(44)正交试验。结果表明,臭氧投加量、催化剂投加量、pH、反应时间是COD去除率及单位臭氧COD降解量的主要影响因素,其中,臭氧投加量与反应时间的影响较为显著。方差分析与试验验证表明,催化剂投加量对COD去除率影响较小,pH对单位臭氧COD降解量影响较小。通过权矩阵计算得到优化后的反应条件:臭氧投加量为5 mg/(L·min),催化剂投加量为0.10 g/L,pH为9,反应时间为45 min。叔丁醇屏蔽试验表明,·OH显著促进了催化臭氧氧化进程。
Abstract:The traditional process has a limited effect on the treatment of phenolic wastewater, and catalytic ozonation technology can effectively treat phenolic wastewater. α-Fe2O3 has exhibited high ozone catalytic activity in previous experiments. ·OH produced by catalysis can non-selectively mineralize phenol and intermediate products, which significantly enhances the removal of pollutants and the level of ozone utilization. In order to clarify the main influencing factors of the catalytic ozone oxidation process and optimize the process parameters, phenol was used to simulate phenolic wastewater, and L16(44) orthogonal experiment was designed. The results showed that ozone dosage, catalyst dosage, pH, and reaction time were the main influencing factors of COD removal rate and COD degradation per unit ozone. Among them, ozone dosage and reaction time had the most significant impact on the two indicators. Variance analysis and experimental verification showed that catalyst dosage had little effect on COD removal rate, and reaction pH had little effect on COD degradation per unit ozone. The optimized process parameters were calculated by weight matrix: ozone dosage was 5 mg/(L·min), catalyst dosage was 0.1 g/L, pH was 9, and reaction time was 45 min. Tert butyl alcohol shielding experiments showed that ·OH significantly promoted the catalytic ozone oxidation process.
-
Key words:
- α-Fe2O3 /
- catalytic ozone oxidation /
- phenol /
- orthogonal experiment /
- weight matrix
-
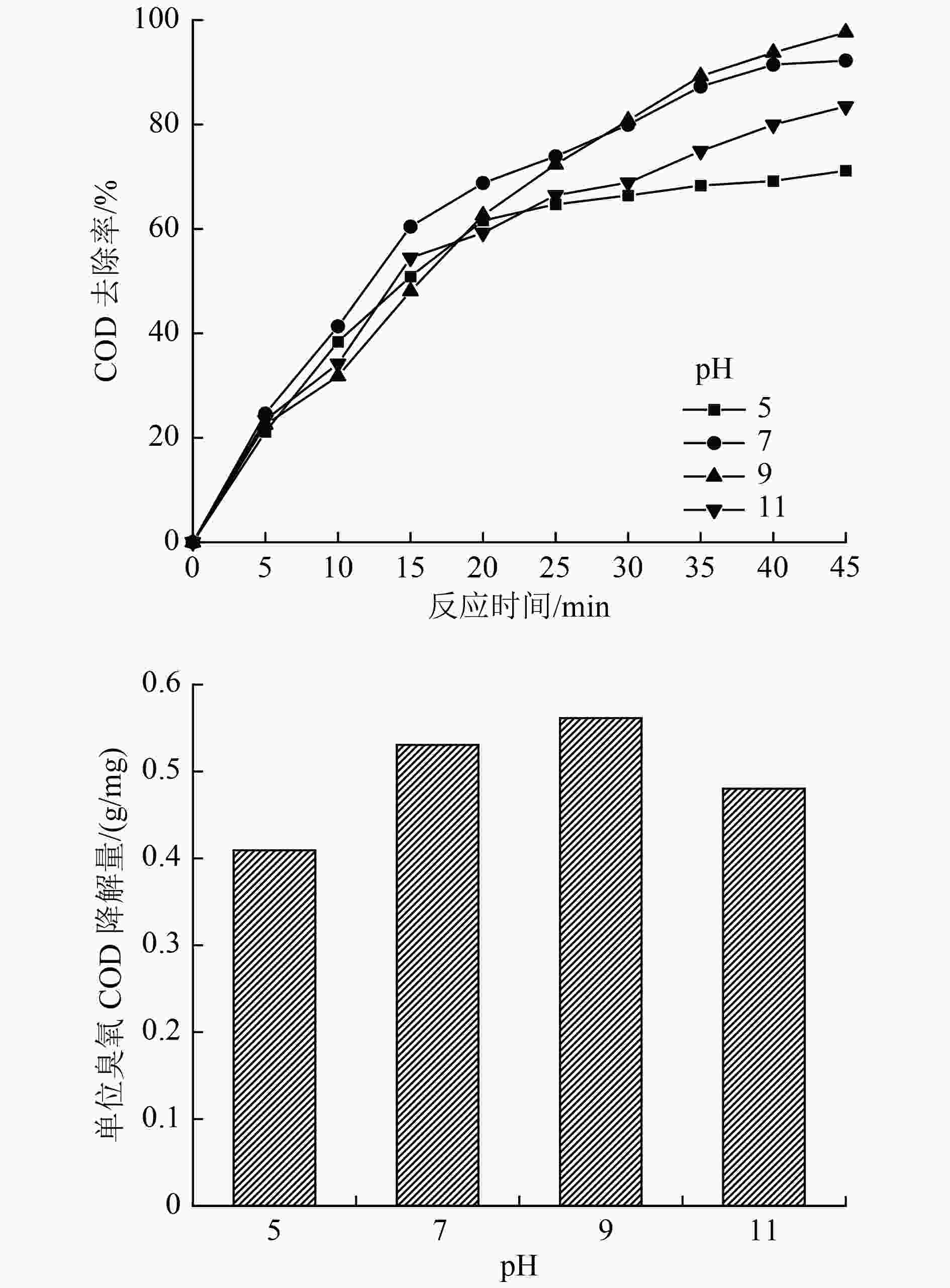
表 1 α-Fe2O3催化臭氧氧化苯酚废水正交试验因素及水平
Table 1. Orthogonal experiment factors and level of α-Fe2O3 catalytic ozonation of phenol wastewater
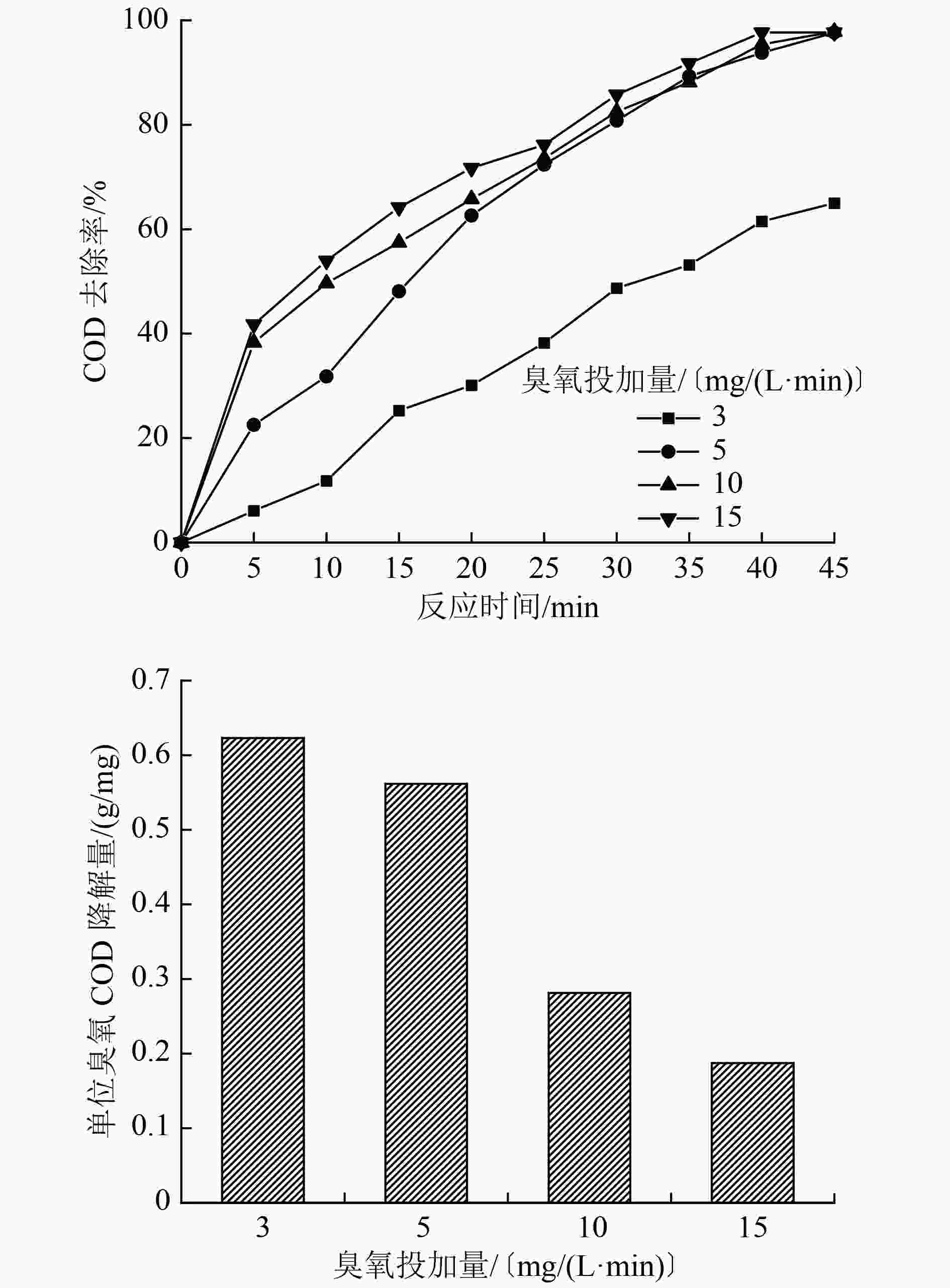
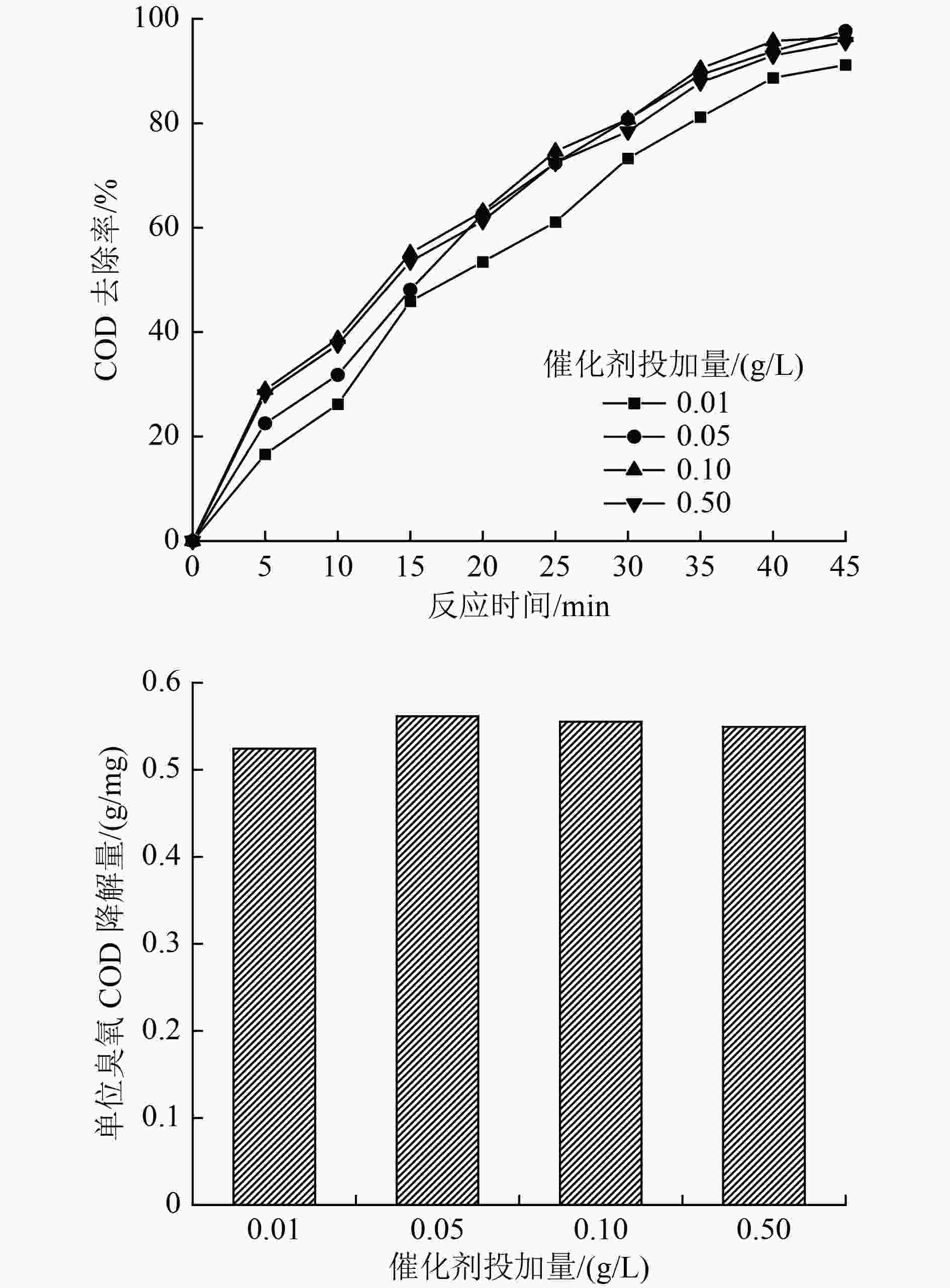
水平 A/〔mg/(L·min)〕 B/(g/L) C D/min 1 3 0.01 5 15 2 5 0.05 7 30 3 10 0.10 9 45 4 13 0.50 11 60 表 2 正交试验设计及试验结果
Table 2. Orthogonal experimental design and experimental results
编号 A B C D COD去除率/% 单位臭氧COD降解量/(g/mg) 1 1 1 1 1 17.68 0.15 2 1 2 2 2 27.89 0.24 3 1 3 3 3 47.67 0.41 4 1 4 4 4 32.54 0.28 5 2 1 1 2 33.71 0.15 6 2 2 3 1 45.34 0.20 7 2 3 2 4 69.76 0.30 8 2 4 4 3 75.58 0.33 9 3 1 2 3 82.56 0.18 10 3 2 4 4 80.23 0.17 11 3 3 1 1 36.04 0.08 12 3 4 3 2 72.09 0.16 13 4 1 3 4 82.56 0.14 14 4 2 1 3 63.95 0.11 15 4 3 4 2 69.76 0.12 16 4 4 2 1 53.48 0.09 表 3 正交试验方差分析
Table 3. Orthogonal test variance analysis
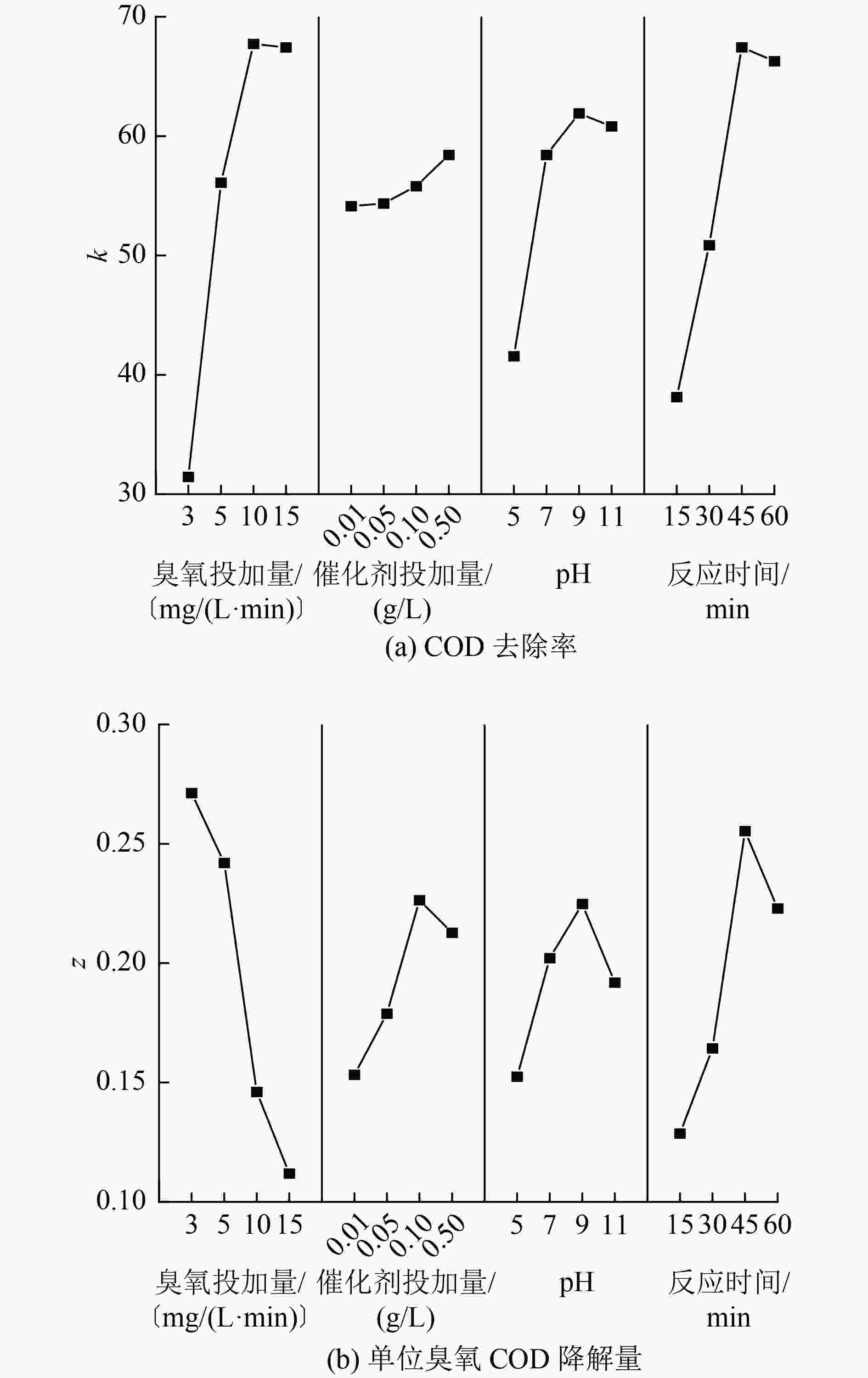
正交参数 A B C D COD去除率 K1 125.78 216.51 166.24 152.53 K2 224.39 217.41 233.69 203.45 K3 270.91 223.22 247.65 269.75 K4 269.74 233.69 243.25 265.09 k1 31.45 54.13 41.56 38.13 k2 56.10 54.35 58.42 50.86 k3 67.73 55.81 61.91 67.44 k4 67.44 58.42 60.81 66.27 R1 36.28 4.29 20.35 29.30 单位臭氧COD降解量 Z1 1.09 0.61 0.61 0.51 Z2 0.97 0.72 0.81 0.66 Z3 0.58 0.91 0.90 1.02 Z4 0.45 0.85 0.77 0.89 z1 0.27 0.15 0.16 0.13 z2 0.24 0.18 0.20 0.16 z3 0.15 0.23 0.22 0.26 z4 0.11 0.21 0.18 0.22 R2 0.16 0.07 0.06 0.13 表 4 影响因素方差分析
Table 4. Variance analysis of influencing factors
指标 试验因素 均方 F 显著性 COD去除率/% A 1 681.46 373.66 ++ B 29.18 6.48 − C 387.27 86.06 ++ D 355.25 78.95 ++ 单位臭氧COD
降解量/(g/mg)A 0.220 50.90 ++ B 0.004 9.95 + C 0.004 8.24 − D 0.013 29.25 ++ 注:+指因素通过0.05的显著性检验;++指因素通过0.01的显著性检验;−指因素影响不显著。 表 5 优化参数条件下试验结果
Table 5. Experimental results under optimized parameters
指标 试验1 试验2 试验3 均值 COD去除率/% 96.83 97.66 97.44 97.31 单位臭氧COD降解量/(g/mg) 0.56 0.56 0.56 0.56 -
[1] ALSHABIB M, ONAIZI S A. A review on phenolic wastewater remediation using homogeneous and heterogeneous enzymatic processes: current status and potential challenges[J]. Separation and Purification Technology,2019,219:186-207. doi: 10.1016/j.seppur.2019.03.028 [2] LI M, CHEN Z Q, WANG Z Z, et al. Investigation on degradation behavior of dissolved effluent organic matter, organic micro-pollutants and bio-toxicity reduction from secondary effluent treated by ozonation[J]. Chemosphere,2019,217:223-231. doi: 10.1016/j.chemosphere.2018.11.039 [3] 李亚男, 谭煜, 吴昌永, 等.臭氧催化氧化在石化废水深度处理应用中的若干问题[J]. 环境工程技术学报,2019,9(3):275-281. doi: 10.12153/j.issn.1674-991X.2019.02.280LI Y N, TAN Y, WU C Y, et al. Application and problems of catalytic ozonation in advanced treatment of petrochemical wastewater[J]. Journal of Environmental Engineering Technology,2019,9(3):275-281. doi: 10.12153/j.issn.1674-991X.2019.02.280 [4] KHUNTIA S, SINHA M K, SINGH P. Theoretical and experimental investigation of the mechanism of the catalytic ozonation process by using a manganese-based catalyst[J]. Environmental Technology,2021,42(4):632-639. doi: 10.1080/09593330.2019.1640800 [5] 陈炜彧, 李旭芳, 马鲁铭.铁基催化剂催化臭氧深度处理煤化工废水[J]. 环境工程学报,2018,12(1):86-92. doi: 10.12030/j.cjee.201706031CHEN W Y, LI X F, MA L M. Advanced treatment of coal chemical wastewater by catalytic ozonation with iron-based catalyst[J]. Chinese Journal of Environmental Engineering,2018,12(1):86-92. doi: 10.12030/j.cjee.201706031 [6] ZHENG Y F, GU X N, WITTE F. Biodegradable metals[J]. Materials Science and Engineering:R:Reports,2014,77:1-34. doi: 10.1016/j.mser.2014.01.001 [7] TRAPIDO M, VERESSININA Y, MUNTER R, et al. Catalytic ozonation of m-dinitrobenzene[J]. Ozone:Science & Engineering,2005,27(5):359-363. [8] HE S M, LI J, XU J, et al. Heterogeneous catalytic ozonation of paper-making wastewater with α-Fe2O3/γ-Al2O3 as a catalyst for increased TOC and color removals[J]. Desalination and Water Treatment,2017,95:192-199. doi: 10.5004/dwt.2017.21535 [9] WANG B, ZHANG H, WANG F F, et al. Application of heterogeneous catalytic ozonation for refractory organics in wastewater[J]. Catalysts,2019,9(3):241. doi: 10.3390/catal9030241 [10] 朱秋实, 陈进富, 姜海洋, 等.臭氧催化氧化机理及其技术研究进展[J]. 化工进展,2014,33(4):1010-1014. doi: 10.3969/j.issn.1000-6613.2014.04.038ZHU Q S, CHEN J F, JIANG H Y, et al. A review of catalytic ozonation: mechanisms and efficiency[J]. Chemical Industry and Engineering Progress,2014,33(4):1010-1014. doi: 10.3969/j.issn.1000-6613.2014.04.038 [11] 王吉坤, 李阳, 陈贵锋, 等.臭氧催化氧化降解煤化工生化进水有机物的实验及机理研究[J]. 化工进展,2021,40(10):5837-5844.WANG J Y, LI Y, CHEN G F, et al. Experimental and mechanism studies on degradation of the organics in biochemical influent of coal chemical industry by ozone catalytic oxidation[J]. Chemical Industry and Engineering Progress,2021,40(10):5837-5844. [12] TRAVAINI R, BARRADO E, BOLADO-RODRÍGUEZ S. Effect of ozonolysis pretreatment parameters on the sugar release, ozone consumption and ethanol production from sugarcane bagasse[J]. Bioresource Technology,2016,214:150-158. doi: 10.1016/j.biortech.2016.04.102 [13] 李光耀, 陈强, 郭文凯, 等.基于正交试验的臭氧及其前体物的非线性响应及控制方案[J]. 环境科学,2021,42(2):616-623. doi: 10.13227/j.hjkx.202007026LI G Y, CHEN Q, GUO W K, et al. Nonlinear response characteristics and control scheme for ozone and its precursors based on orthogonal experimental methods[J]. Environmental Science,2021,42(2):616-623. doi: 10.13227/j.hjkx.202007026 [14] KNOWLES S L, VU N, TODD D A, et al. Orthogonal method for double-bond placement via ozone-induced dissociation mass spectrometry (OzID-MS)[J]. Journal of Natural Products,2019,82(12):3421-3431. doi: 10.1021/acs.jnatprod.9b00787 [15] 季伟伟, 杨慧中.基于正交实验的水质COD在线测试最优消解条件[J]. 环境工程学报,2016,10(7):3967-3972. doi: 10.12030/j.cjee.201501227JI W W, YANG H Z. Optimum digestion conditions for on-line monitoring of COD in water based on orthogonal experimental method[J]. Chinese Journal of Environmental Engineering,2016,10(7):3967-3972. doi: 10.12030/j.cjee.201501227 [16] JUYBARI M N, GUILANI P P, ARDAKAN M A. Bi-objective sequence optimization in reliability problems with a matrix-analytic approach[J]. Annals of Operations Research,2021:1-30. [17] RAHMAN M, MAHMOOD A, YOUNIS M. Improved and more feasible numerical methods for Riesz space fractional partial differential equations[J]. Applied Mathematics and Computation,2014,237:264-273. doi: 10.1016/j.amc.2014.03.103 [18] MOHAMMADI L, BAZRAFSHAN E, NOROOZIFAR M, et al. Removing 2, 4-dichlorophenol from aqueous environments by heterogeneous catalytic ozonation using synthesized MgO nanoparticles[J]. Water Science and Technology,2017,76(11):3054-3068. doi: 10.2166/wst.2017.479 [19] WU Z W, XU X C, JIANG H B, et al. Evaluation and optimization of a pilot-scale catalytic ozonation-persulfate oxidation integrated process for the pretreatment of dry-spun acrylic fiber wastewater[J]. RSC Advances,2017,7(70):44059-44067. doi: 10.1039/C7RA03287K [20] 许珊珊, 林存旺, 丁亚磊, 等.MgO/活性炭催化臭氧化降解有机物的作用机制[J]. 环境科学,2018,39(2):838-843. doi: 10.13227/j.hjkx.201704082XU S S, LIN C W, DING Y L, et al. Mechanism of MgO/GAC catalyzed ozonation of organic compounds[J]. Environmental Science,2018,39(2):838-843. doi: 10.13227/j.hjkx.201704082 [21] HUANG Y X, CUI C C, ZHANG D F, et al. Heterogeneous catalytic ozonation of dibutyl phthalate in aqueous solution in the presence of iron-loaded activated carbon[J]. Chemosphere,2015,119:295-301. doi: 10.1016/j.chemosphere.2014.06.060 [22] QI F, CHU W, XU B B. Ozonation of phenacetin in associated with a magnetic catalyst CuFe2O4: the reaction and transformation[J]. Chemical Engineering Journal,2015,262:552-562. doi: 10.1016/j.cej.2014.09.068 [23] WANG J L, BAI Z Y. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater[J]. Chemical Engineering Journal,2017,312:79-98. doi: 10.1016/j.cej.2016.11.118 [24] MANSAS C, MENDRET J, BROSILLON S, et al. Coupling catalytic ozonation and membrane separation: a review[J]. Separation and Purification Technology,2020,236:116221. doi: 10.1016/j.seppur.2019.116221 [25] WANG B, XIONG X, REN H Y, et al. Preparation of MgO nanocrystals and catalytic mechanism on phenol ozonation[J]. RSC Advances,2017,7(69):43464-43473. doi: 10.1039/C7RA07553G [26] JIAO W Z, SHAO S J, YANG P Z, et al. Kinetics and mechanism of nitrobenzene degradation by hydroxyl radicals-based ozonation process enhanced by high gravity technology[J]. Frontiers of Chemical Science and Engineering,2021,15(5):1197-1205. doi: 10.1007/s11705-020-1998-6 [27] NÖTHE T, FAHLENKAMP H, von SONNTAG C. Ozonation of wastewater: rate of ozone consumption and hydroxyl radical yield[J]. Environmental Science & Technology,2009,43(15):5990-5995. [28] ZHANG S, QUAN X E, WANG D. Catalytic ozonation in arrayed zinc oxide nanotubes as highly efficient mini-column catalyst reactors (MCRs): augmentation of hydroxyl radical exposure[J]. Environmental Science & Technology,2018,52(15):8701-8711. [29] LI Y, WU L C, WANG Y, et al. Γ-Al2O3 doped with cerium to enhance electron transfer in catalytic ozonation of phenol[J]. Journal of Water Process Engineering,2020,36:101313. doi: 10.1016/j.jwpe.2020.101313 [30] 任越中, 张嘉雯, 魏健, 等.铈负载改性天然沸石催化臭氧氧化水中青霉素G[J]. 环境工程技术学报,2019,9(1):28-35. doi: 10.3969/j.issn.1674-991X.2019.01.005REN Y Z, ZHANG J W, WEI J, et al. Catalytic ozonation of penicillin G in aqueous phase using modified natural zeolite supported cerium[J]. Journal of Environmental Engineering Technology,2019,9(1):28-35. □ doi: 10.3969/j.issn.1674-991X.2019.01.005 -




 下载:
下载: