Preparation of modified anthracites and research on their adsorption and recovery performance on phosphorus
-
摘要:
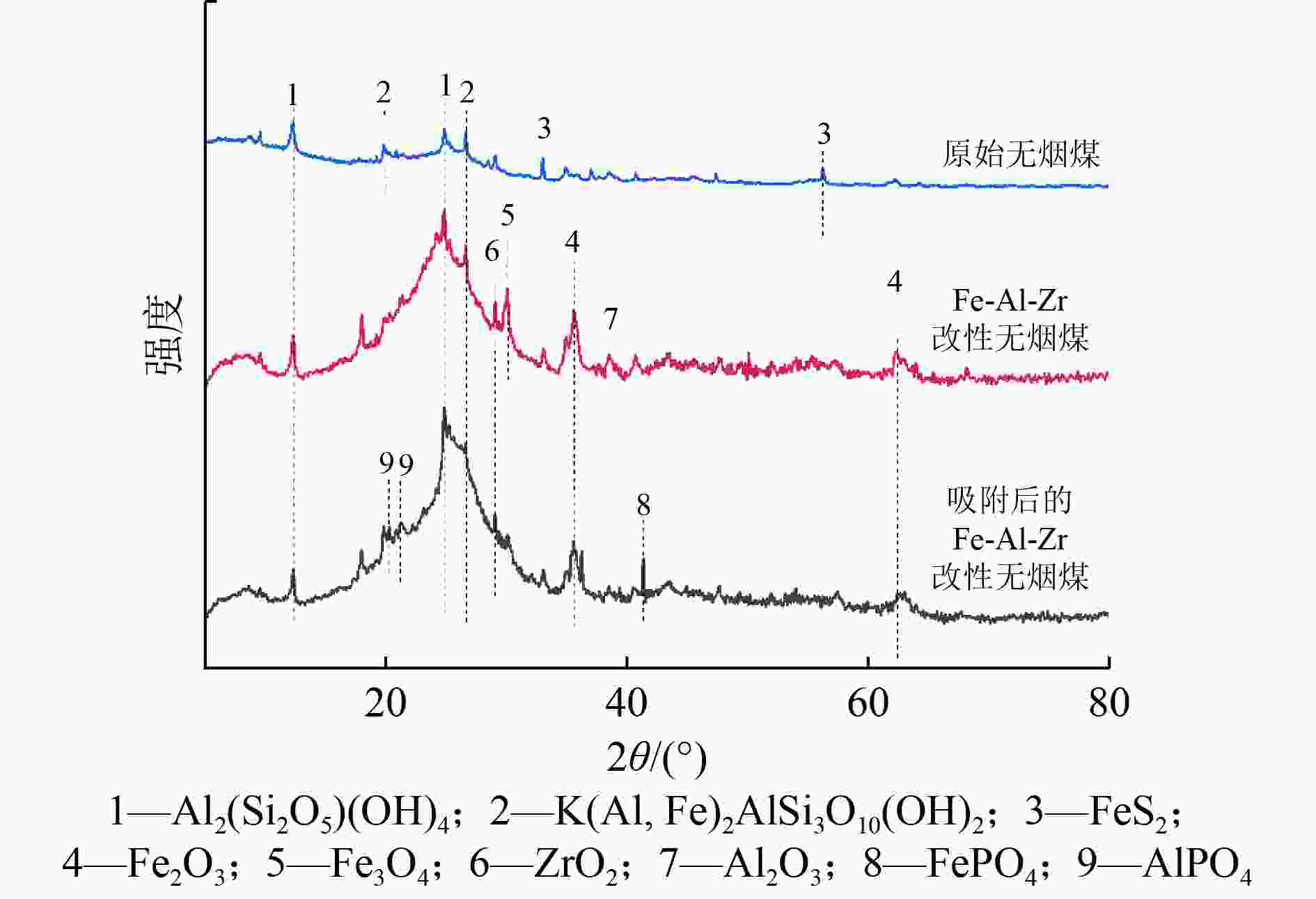
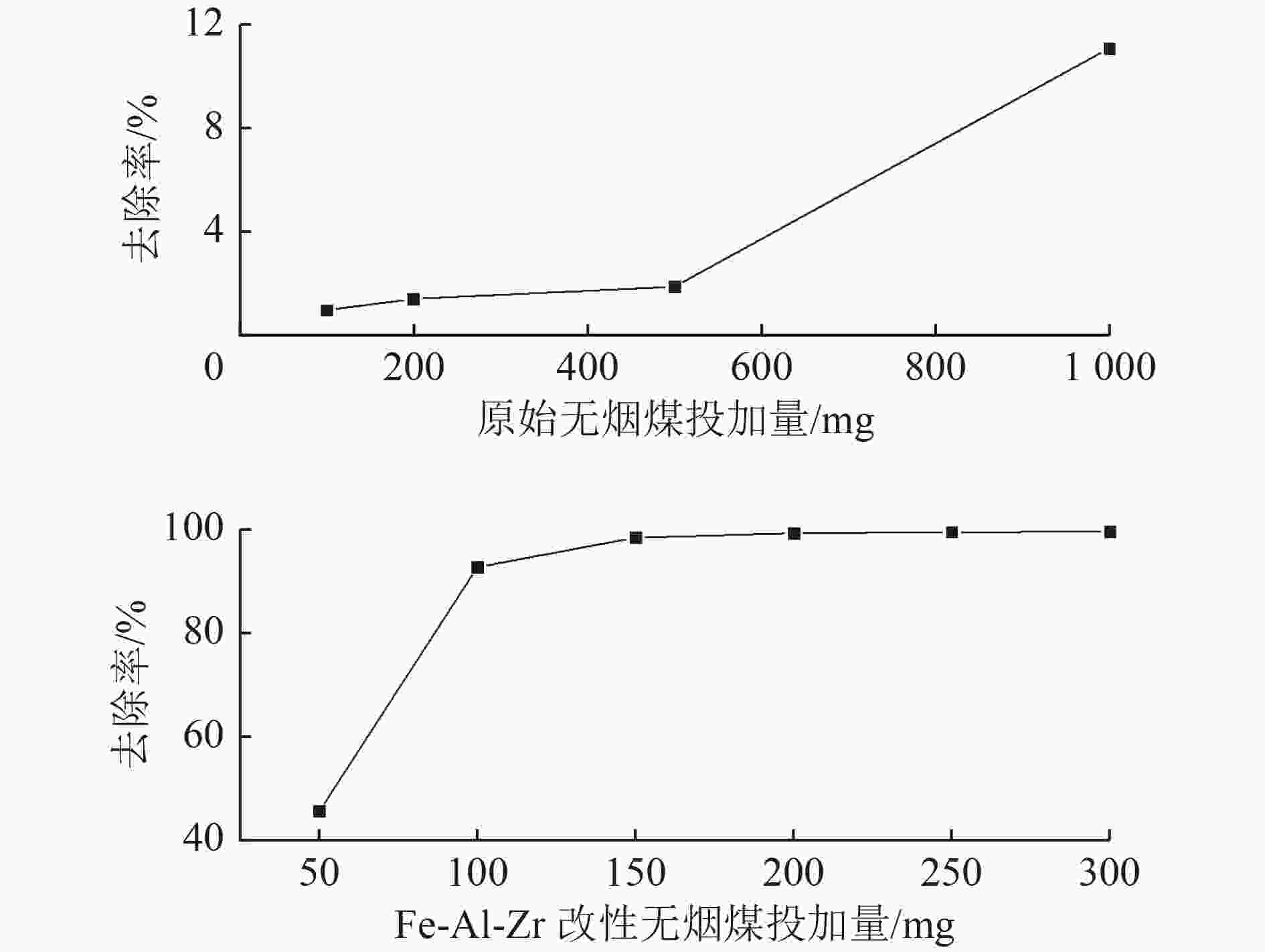
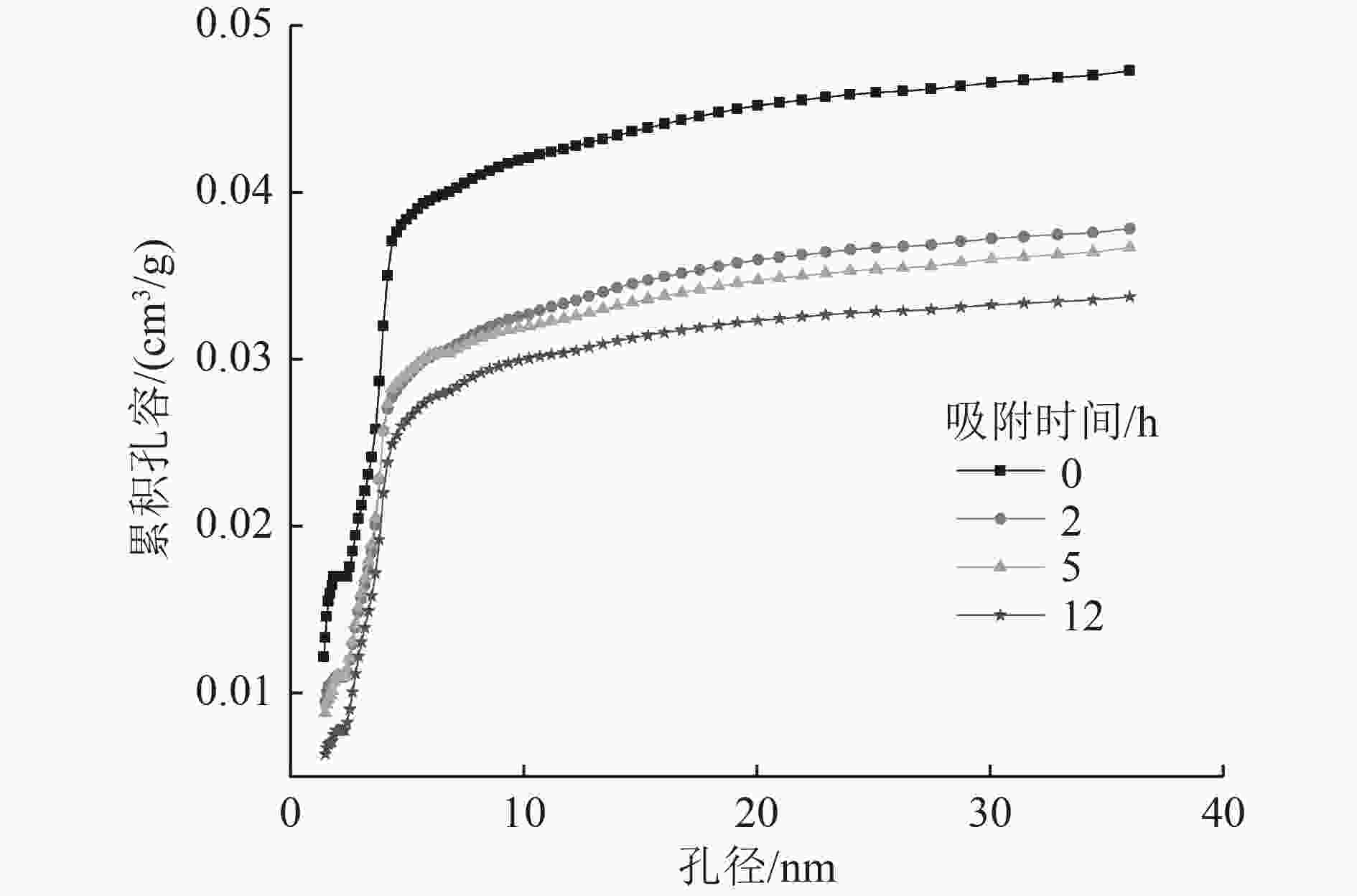
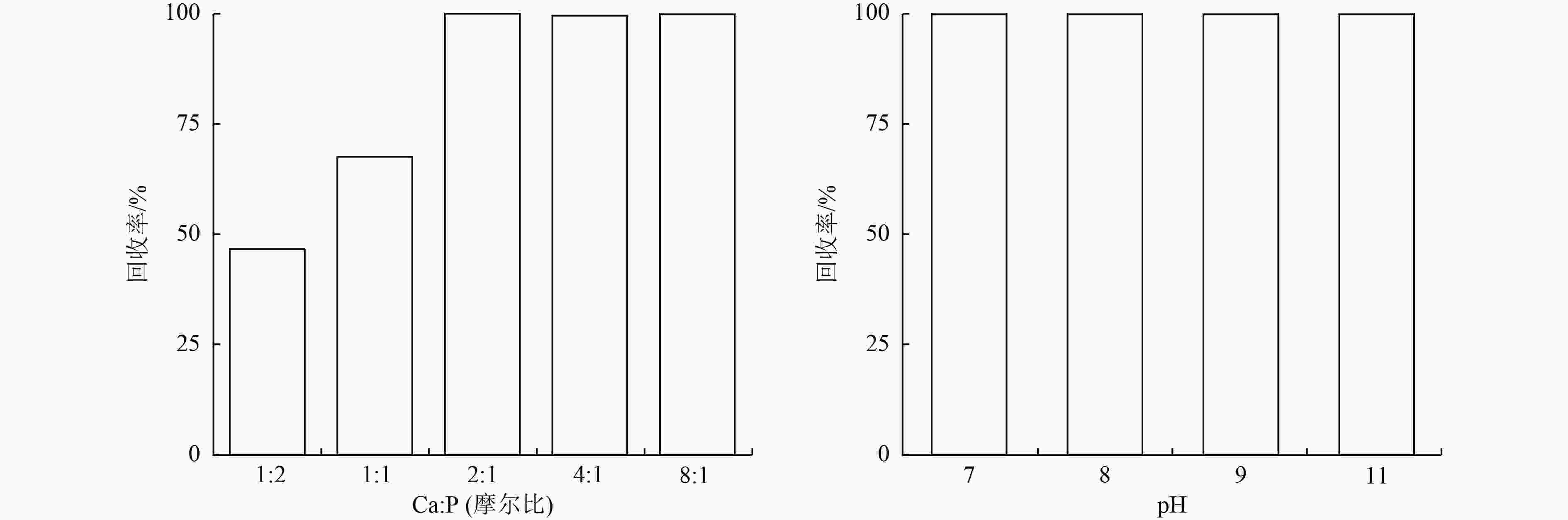
为了实现污水中磷的回收与资源化利用,提出采用Fe-Al-Zr改性的无烟煤材料吸附-回收磷的方法。该吸附剂对磷的总吸附量为13.022 mg/g,吸附机理主要包括静电作用、配体交换和表面沉积等;微孔提供主要的吸附位点,决定了磷的吸附容量。该吸附剂可循环使用4个周期,直至磷的吸附率低于50%。在碱性条件下,通过投加一定量的CaCl2〔Ca∶P(摩尔比)=2∶1〕,磷能够以羟基磷灰石(HAP)的形式脱附和被回收。
-
关键词:
- Fe-Al-Zr改性 /
- 无烟煤 /
- 吸附机理 /
- 磷回收 /
- 羟基磷灰石(HAP)
Abstract:To recover and reutilize phosphorus from wastewater, a methodological framework for phosphorous removal and recovery using Fe-Al-Zr modified anthracite materials was proposed. The adsorbent presented a total adsorption capacity of 13.022 mg/g and the adsorption mechanism mainly included electrostatic forces, ligands exchange and surface deposition and so on. Micropores provided the major adsorption sites for phosphorous, and determined the adsorption capacity of phosphorous. The modified adsorbents could be repeatedly used in four operation cycles until the adsorption rate decreased below 50%. The phosphorous could be desorbed and recovered in the form of hydroxyapatite (HAP) by adding a certain amount of CaCl2 (n(Ca)∶n(P)=2∶1) under alkaline conditions.
-
Key words:
- Fe-Al-Zr modification /
- anthracite /
- adsorption mechanism /
- phosphorus recovery /
- hydroxyapatite (HAP)
-
表 1 无烟煤改性过程中药剂投加量的正交设计
Table 1. Orthogonal design of the dosage of reagent in the anthracite modification process
药剂投加量(mmol/g,以无烟煤计) 磷的去
除率/%FeSO4·7H2O FeCl3·6H2O Cl2OZr·8H2O Al(NO3)3·9H2O 1 2 2 2 71.35 1 2 4 4 93.37 2 4 4 4 68.79 2 4 4 2 63.87 表 2 Fe-Al-Zr改性无烟煤吸附磷的动力学与等温吸附模型拟合参数
Table 2. Fitting parameters of kinetic models and isotherm parameters for the adsorption of phosphorus onto Fe-Al-Zr modified anthracites
模型 模型参数 拟合值 伪一级动力学模型
${q_t}{\text{ = } }{q_{\rm{e} } }(1 - { {\rm{e} }^{ - {K_1}t} })$K1/(min−1) 0.008 6 qe/(mg/g) 3.256 R2 0.975 1 伪二级动力学模型
$\dfrac{t}{ { {q_t} } } = \dfrac{1}{ { {K_2}q_{\rm{e}}^2} } + \dfrac{t}{ { {q_{\rm{e}}} } }$K2/〔g/(mg·min)〕 0.006 0 qe/(mg/g) 5.656 R2 0.998 8 Langmuir模型
${q_{\rm{e}}} = \dfrac{ { {q_{\rm{m}}}{K_{\rm{L}}}{C_{\rm{e}}} } }{ {1 + {K_{\rm{L}}}{C_{\rm{e}}} } }$qm/(mg/g) 12.853 0 KL (L/mg) 2.350 R2 0.998 3 Freundlich模型
${q_{\rm{e}}} = {K_{\rm{F}}}{C_{\rm{e}}}^{1/n}$KF/〔(mg/g)/(mg/L)1/n〕 6.373 0 1/n 2.378 R2 0.918 2 表 3 Fe-Al-Zr改性无烟煤吸附磷的颗粒内扩散模型拟合参数
Table 3. Fitting parameters of intraparticle diffusion model for the adsorption of phosphorus onto Fe-Al-Zr modified anthracites
模型 参数 第一阶段 第二阶段 $ {q}_{t}={K}_{i}{t}^{1/2}+C $ Ki/〔mg/(g·min−1/2)〕 0.188 0.009 C/(mg/g) 2.012 5.138 R2 0.978 7 0.648 1 表 4 不同吸附剂除磷性能的比较
Table 4. Comparison of phosphorous adsorption of different adsorbent
表 5 Fe-Al-Zr改性无烟煤在4个循环周期中对磷的吸附量和去除率
Table 5. Adsorption amount and removal efficiency of Fe-Al-Zr modified anthracites in the four operation cycles
项目 第1次循环 第2次循环 第3次循环 第4次循环 第1次吸附 第2次吸附 第3次吸附 第1次吸附 第2次吸附 第1次吸附 第2次吸附 第1次吸附 吸附量/(mg/g) 6.289 4.181 2.552 4.915 2.139 3.645 1.309 2.778 去除率/% 96.42 76.53 44.12 76.53 35.28 60.93 22.48 48.03 -
[1] 桑倩倩, 王芳君, 赵元添, 等.铁硫改性生物炭去除水中的磷[J]. 环境科学,2021,42(5):2313-2323.SANG Q Q, WANG F J, ZHAO Y T, et al. Application of iron and sulfate-modified biochar in phosphorus removal from water[J]. Environmental Science,2021,42(5):2313-2323. [2] 崔荣国, 张艳飞, 郭娟, 等.资源全球配置下的中国磷矿发展策略[J]. 中国工程科学,2019,21(1):128-132.CUI R G, ZHANG Y F, GUO J, et al. Development strategy of phosphate rock in China under global allocation of resources[J]. Engineering Science,2019,21(1):128-132. [3] SHEPHERD J G, SOHI S P, HEAL K V. Optimising the recovery and re-use of phosphorus from wastewater effluent for sustainable fertiliser development[J]. Water Research,2016,94:155-165. doi: 10.1016/j.watres.2016.02.038 [4] LIU T, CHEN X, WANG X, et al. Highly effective wastewater phosphorus removal by phosphorus accumulating organism combined with magnetic sorbent MFC@La(OH)3[J]. Chemical Engineering Journal,2018,335:443-449. doi: 10.1016/j.cej.2017.10.117 [5] 赵鹏, 李红艳, 崔建国, 等.载铝活性炭纤维的制备及其脱氮除磷性能[J]. 工业水处理,2020,40(11):79-83.ZHAO P, LI H Y, CUI J G, et al. Preparation of aluminum-loaded activated carbon fiber and its nitrogen and phosphorus removal performance[J]. Industrial Water Treatment,2020,40(11):79-83. [6] OGINNI O, YAKABOYLU G A, SINGH K, et al. Phosphorus adsorption behaviors of MgO modified biochars derived from waste woody biomass resources[J]. Journal of Environmental Chemical Engineering,2020,8(2):103723. doi: 10.1016/j.jece.2020.103723 [7] 王春丽, 吴俊奇, 宋永会, 等.活化赤泥颗粒吸附除磷的效能与机制研究[J]. 环境工程技术学报,2015,5(2):143-148. doi: 10.3969/j.issn.1674-991X.2015.02.021WANG C L, WU J Q, SONG Y H, et al. Research on performance and mechanisms of activated red mud particles on adsorbing and removing phosphorus[J]. Journal of Environmental Engineering Technology,2015,5(2):143-148. doi: 10.3969/j.issn.1674-991X.2015.02.021 [8] 何一帆, 聂小保, 余志, 等.低磷污水的HAP诱导结晶磷回收[J]. 环境科学学报,2021,41(2):566-573.HE Y F, NIE X B, YU Z, et al. Phosphorus recovery from wastewater with low phosphorus concentration by HAP induced crystallization[J]. Acta Scientiae Circumstantiae,2021,41(2):566-573. [9] 胡怡, 宋永会, 钱锋, 等.赤泥诱导磷酸钙结晶法回收废水中的磷[J]. 环境工程技术学报,2014,4(1):60-66. doi: 10.3969/j.issn.1674-991X.2014.01.011HU Y, SONG Y H, QIAN F, et al. Phosphorus recovery from wastewater by red mud-seeded crystallization of calcium phosphate[J]. Journal of Environmental Engineering Technology,2014,4(1):60-66. doi: 10.3969/j.issn.1674-991X.2014.01.011 [10] LEI Y, SAAKES M, van der WEIJDEN R D, et al. Electrochemically mediated calcium phosphate precipitation from phosphonates: implications on phosphorus recovery from non-orthophosphate[J]. Water Research,2020,169:115206. doi: 10.1016/j.watres.2019.115206 [11] HE J, ZHOU Q H, GUO J S, et al. Incredulity on assumptions for the simplified Bohart-Adams model: 17a-ethinylestradiol separation in lab-scale anthracite columns[J]. Journal of Hazardous Materials,2020,384:121501. doi: 10.1016/j.jhazmat.2019.121501 [12] PAI C W, LEONG D, CHEN C Y, et al. Occurrences of pharmaceuticals and personal care products in the drinking water of Taiwan and their removal in conventional water treatment processes[J]. Chemosphere,2020,256:127002. doi: 10.1016/j.chemosphere.2020.127002 [13] MOHAMED E A, SELIM A Q, AHMED S A, et al. H2O2-activated anthracite impregnated with chitosan as a novel composite for Cr(Ⅵ) and methyl orange adsorption in single-compound and binary systems: modeling and mechanism interpretation[J]. Chemical Engineering Journal,2020,380:122445. doi: 10.1016/j.cej.2019.122445 [14] 常会庆, 王浩, 徐晓峰.无烟煤活性炭对酸碱性不同染料废水的吸附研究[J]. 水土保持学报,2014,28(2):276-280.CHANG H Q, WANG H, XU X F. Adsorption studies of acid and base dyes wastewater on anthracite activated carbon[J]. Journal of Soil and Water Conservation,2014,28(2):276-280. [15] 简志强, 周高婷, 龚斌, 等.微米零价铁去除磷酸盐效果与机理研究[J]. 环境工程技术学报,2021,11(5):927-934. doi: 10.12153/j.issn.1674-991X.20210027JIAN Z Q, ZHOU G T, GONG B, et al. Study on the efficacy of micron zero-valent iron on phosphate removal and its mechanism[J]. Journal of Environmental Engineering Technology,2021,11(5):927-934. doi: 10.12153/j.issn.1674-991X.20210027 [16] FENG J W, JIANG L, YUAN B X, et al. Enhanced removal of aqueous phosphorus by Zr–Fe-, Mn–Fe-, and Mn–Zr–Fe-modified natural zeolites: comparison studies and adsorption mechanism[J]. Environmental Engineering Science,2020,37(8):572-584. doi: 10.1089/ees.2019.0490 [17] LIN X C, XIE Y L, LU H J, et al. Facile preparation of dual La-Zr modified magnetite adsorbents for efficient and selective phosphorus recovery[J]. Chemical Engineering Journal,2021,413:127530. doi: 10.1016/j.cej.2020.127530 [18] HE J, GUO J S, ZHOU Q H, et al. Adsorption characteristics of nitrite on natural filter medium: Kinetic, equilibrium, and site energy distribution studies[J]. Ecotoxicology and Environmental Safety,2019,169:435-441. doi: 10.1016/j.ecoenv.2018.11.039 [19] WANG M, YU X L, YANG C L, et al. Removal of fluoride from aqueous solution by Mg-Al-Zr triple-metal composite[J]. Chemical Engineering Journal,2017,322:246-253. doi: 10.1016/j.cej.2017.03.155 [20] MACÍAS-GARCÍA A, GÓMEZ CORZO M, ALFARO DOMÍNGUEZ M, et al. Study of the adsorption and electroadsorption process of Cu(Ⅱ) ions within thermally and chemically modified activated carbon[J]. Journal of Hazardous Materials,2017,328:46-55. doi: 10.1016/j.jhazmat.2016.11.036 [21] 刘赫尊, 陈亮, 张海丰, 等.改性海绵铁深度除磷及其再生磷回收方法[J]. 环境科学学报,2020,40(1):147-154.LIU H Z, CHEN L, ZHANG H F, et al. Phosphorus removal and recovery based on modified sponge iron[J]. Acta Scientiae Circumstantiae,2020,40(1):147-154. [22] LI Y G, LI Q Q, WU C X, et al. The inappropriate application of the regression Langmuir Qm for adsorption capacity comparison[J]. Science of the Total Environment,2020,699:134222. doi: 10.1016/j.scitotenv.2019.134222 [23] 赵敏, 张小平, 王梁嵘. 2021. 硅改性花生壳生物炭对水中磷的吸附特性[J/OL]. 环境科学, 2021. doi: 10.13227/j.hjkx.202103012.ZHAO M, ZHANG X P, WANG L R. Characteristics of phosphorous adsorption in aqueous solution by Si-modified peanut shell biochar[J/OL]. Environmental Science, 2021. doi: 10.13227/j.hjkx.202103012. [24] 梁宁, 莫福金, 周街荣, 等.污泥生物炭制备及其对磷的吸附性能研究[J]. 无机盐工业,2021,53(6):174-179.LIANG N, MO F J, ZHOU J R, et al. Study on preparation of sludge biochar and its adsorption performance for phosphorus[J]. Inorganic Chemicals Industry,2021,53(6):174-179. [25] 肖作义, 肖宇, 肖明慧, 等.磁性水滑石/生物炭复合材料的制备及其对水溶液中磷的吸附性能[J]. 环境污染与防治,2020,42(9):1090-1095. doi: 10.15985/j.cnki.1001-3865.2020.09.005XIAO Z Y, XIAO Y, XIAO M H, et al. Preparation of magnetic hydrotalcite/biochar composite and its adsorption performance for phosphorus in aqueous solution[J]. Environmental Pollution & Control,2020,42(9):1090-1095. doi: 10.15985/j.cnki.1001-3865.2020.09.005 [26] 张晓, 陈晨, 程婷, 等.微孔碳@粉煤灰颗粒复合材料合成及其吸附磷应用[J]. 有色金属工程,2020,10(9):134-144. doi: 10.3969/j.issn.2095-1744.2020.09.020ZHANG X, CHEN C, CHENG T, et al. Synthesis of micro-porous Carbon@Fly ash particle composite material and its application to phosphorus adsorption[J]. Nonferrous Metals Engineering,2020,10(9):134-144. doi: 10.3969/j.issn.2095-1744.2020.09.020 [27] YE J E, CONG X N, ZHANG P Y, et al. Interaction between phosphate and acid-activated neutralized red mud during adsorption process[J]. Applied Surface Science,2015,356:128-134. □ doi: 10.1016/j.apsusc.2015.08.053 -




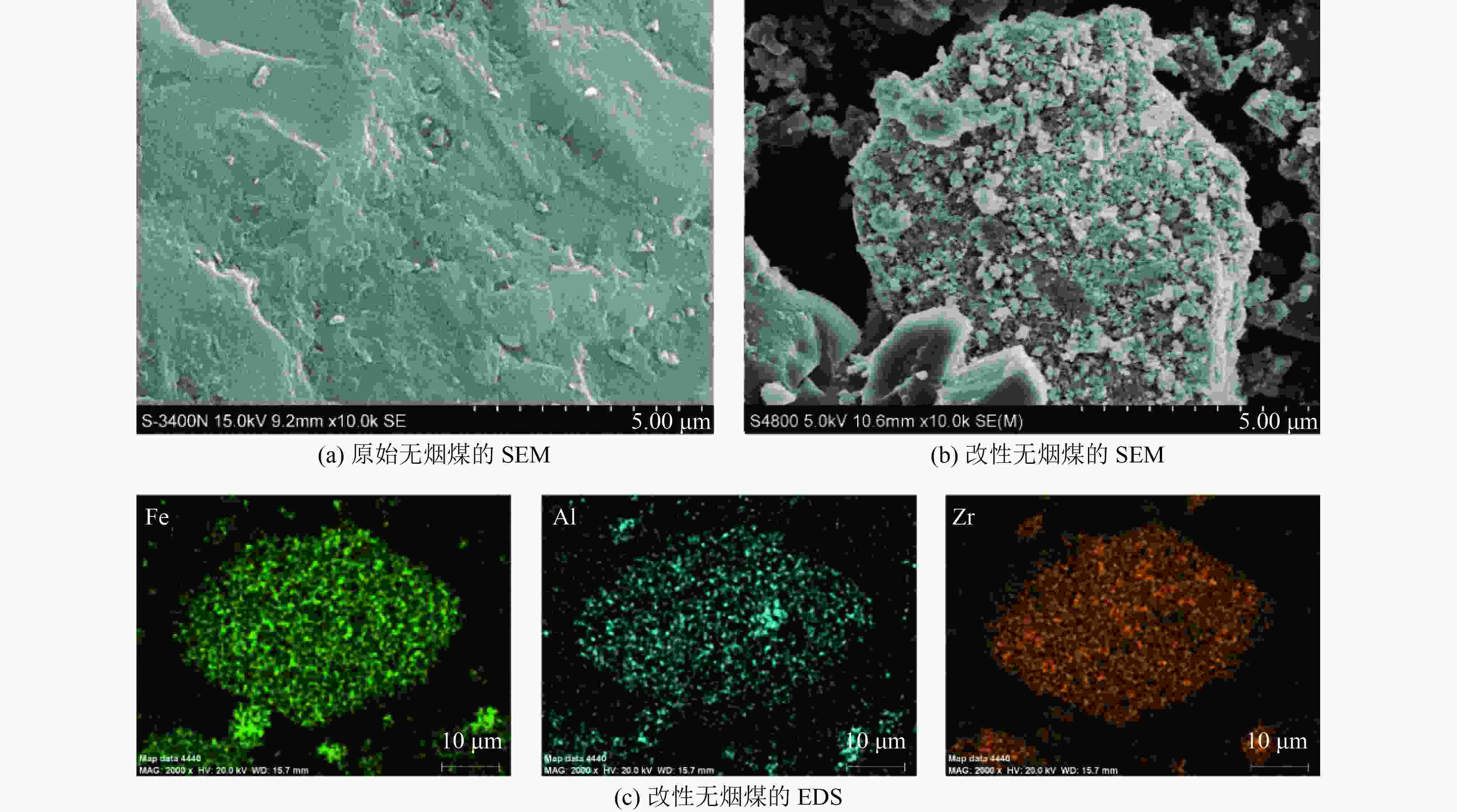
 下载:
下载: