Research progress on heavy metals removal from wastewater by biochar-supported nano zero-valent iron
-
摘要:
纳米零价铁(nZVI)因比表面积大、反应活性高及独特的核壳结构,在去除水中重金属方面具有良好的应用前景。但nZVI自身存在易团聚、易氧化失活等缺点,使其工业推广和应用受到限制。将nZVI负载于生物炭(BC)制备生物炭负载型纳米零价铁(nZVI@BC)复合材料可在一定程度上克服nZVI的缺点,提高nZVI与重金属的反应活性。综述了nZVI@BC去除水中重金属的研究现状,着重介绍了不同BC材料用于nZVI@BC的制备、BC的改性及nZVI的修饰对nZVI@BC去除重金属性能的影响,阐述了nZVI@BC去除几种典型重金属的反应机理,并对nZVI@BC应用于水中重金属去除的发展前景进行了展望。
-
关键词:
- 生物炭 /
- 纳米零价铁(nZVI) /
- 改性 /
- 重金属 /
- 机理
Abstract:Nano zero-valent iron (nZVI) has a large specific surface area, high reactivity and unique core-shell structure, which has a good application prospect in the removal of heavy metals in water. However, nZVI itself has some disadvantages such as easy agglomeration and oxidation deactivation, and its industrial promotion and application are limited. The biochar-supported nano-zero-valent iron (nZVI@BC) composite prepared by loading nZVI on biochar (BC) can overcome the shortcomings of nZVI to a certain extent and improve the reaction activity of nZVI with heavy metals. The research status of nZVI@BC removal of heavy metals from water was reviewed. The preparation of nZVI@BC with different BC materials, the modification of BC and the influence of nZVI modification on the removal performance of heavy metals from nZVI@BC were emphatically introduced. The reaction mechanism of nZVI@BC removal of several typical heavy metals was described. The development prospect of nZVI@BC in removing heavy metals from water was also prospected.
-
Key words:
- biochar /
- nano zero valent iron(nZVI) /
- modify /
- heavy metals /
- mechanism
-
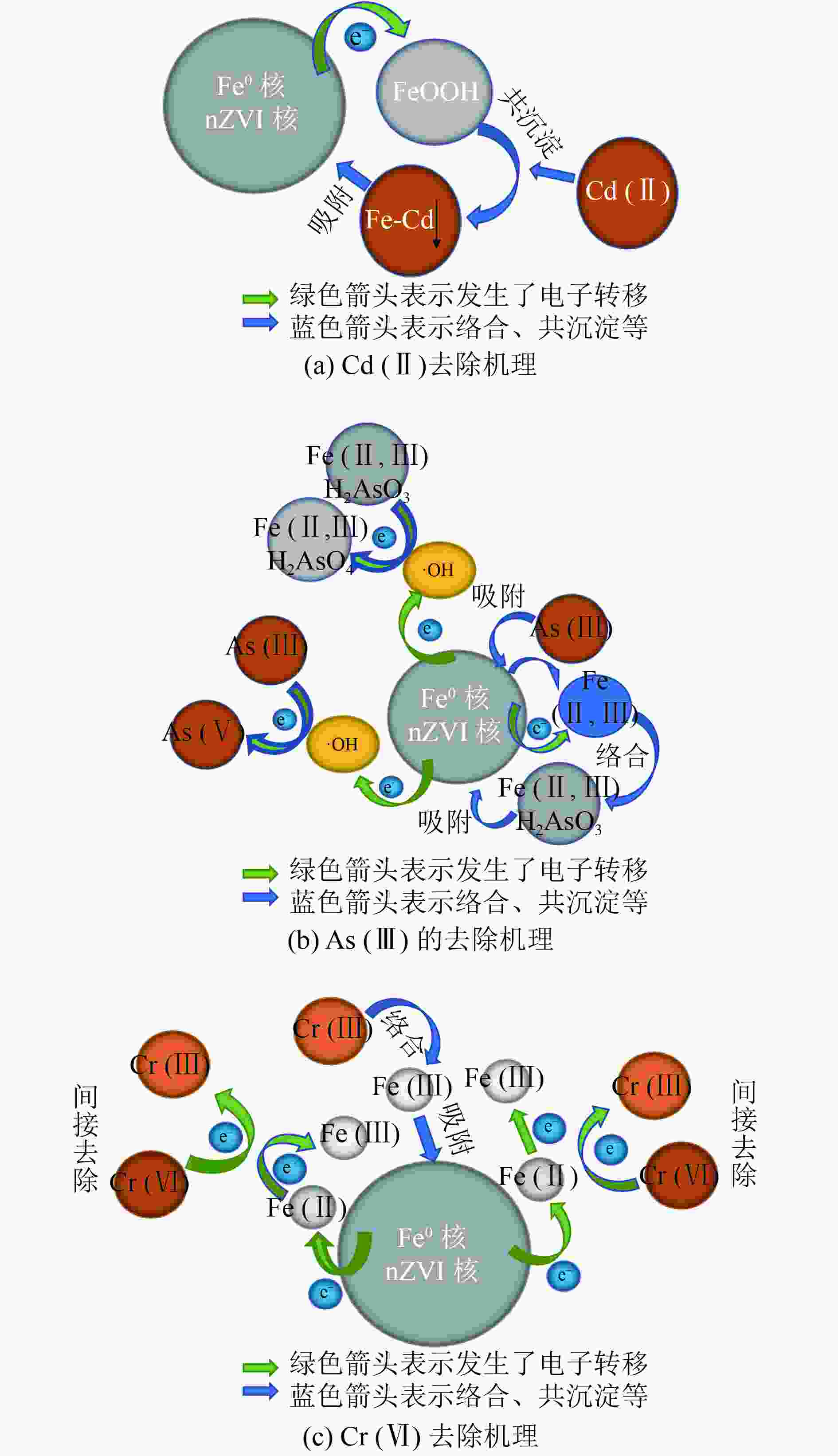
表 1 不同BC制备的nZVI@BC对水溶液中重金属的最大吸附容量
Table 1. Maximum adsorption capacity of heavy metals by nZVI@BC prepared by different BC in aqueous solution
表 2 不同化学方法改性BC制备的nZVI@BC对重金属的吸附容量
Table 2. Adsorption capacity of heavy metals by nZVI@BC with BC modified by different chemical methods
-
[1] YU Y, AN Q, JIN L, et al. Unraveling sorption of Cr(Ⅵ) from aqueous solution by FeCl3 and ZnCl2-modified corn stalks biochar: implicit mechanism and application[J]. Bioresource Technology,2020,297:122466. doi: 10.1016/j.biortech.2019.122466 [2] PEI G P, ZHU Y E, WEN J G, et al. Vinegar residue supported nanoscale zero-valent iron: remediation of hexavalent chromium in soil[J]. Environmental Pollution,2020,256:113407. doi: 10.1016/j.envpol.2019.113407 [3] ZHU L, TONG L H, ZHAO N, et al. Coupling interaction between porous biochar and nano zero valent iron/nano α-hydroxyl iron oxide improves the remediation efficiency of cadmium in aqueous solution[J]. Chemosphere,2019,219:493-503. doi: 10.1016/j.chemosphere.2018.12.013 [4] FAN J, CHEN X, XU Z B, et al. One-pot synthesis of nZVI-embedded biochar for remediation of two mining arsenic-contaminated soils: arsenic immobilization associated with iron transformation[J]. Journal of Hazardous Materials,2020,398:122901. doi: 10.1016/j.jhazmat.2020.122901 [5] 廖家隆, 张喆秋, 陈丽杰, 等.含砷废水处理研究进展[J]. 有色金属科学与工程,2018,9(1):86-91.LIAO J L, ZHANG Z Q, CHEN L J, et al. Research progress of arsenic-containing wastewater treatment[J]. Nonferrous Metals Science and Engineering,2018,9(1):86-91. [6] ZHANG J S, CHEN S J, ZHANG H W, et al. Removal behaviors and mechanisms of hexavalent chromium from aqueous solution by cephalosporin residue and derived chars[J]. Bioresource Technology,2017,238:484-491. doi: 10.1016/j.biortech.2017.04.081 [7] 张慧, 李宁, 戴友芝.重金属污染的生物修复技术[J]. 化工进展,2004,23(5):562-565. doi: 10.3321/j.issn:1000-6613.2004.05.024ZHANG H, LI N, DAI Y Z. Research about the bioremediation of heavy metal pollution[J]. Chemical Industry and Engineering Progress,2004,23(5):562-565. doi: 10.3321/j.issn:1000-6613.2004.05.024 [8] 黄熙贤. 生物炭改性材料对水体Cr(Ⅵ)的吸附还原机理研究[D]. 长沙: 湖南大学, 2017. [9] LIU C M, DIAO Z H, HUO W Y, et al. Simultaneous removal of Cu2+ and bisphenol A by a novel biochar-supported zero valent iron from aqueous solution: synthesis, reactivity and mechanism[J]. Environmental Pollution,2018,239:698-705. doi: 10.1016/j.envpol.2018.04.084 [10] 张常安, 金蕾, 黄应平, 等.丙酮冲洗法提高纳米零价铁抗氧化性能[J]. 环境化学,2021,40(3):790-798. doi: 10.7524/j.issn.0254-6108.2020081808ZHANG C A, JIN L, HUANG Y P, et al. Anti-oxidation ability of nanoscale zero valent iron viaacetone flushing method[J]. Environmental Chemistry,2021,40(3):790-798. doi: 10.7524/j.issn.0254-6108.2020081808 [11] 严子春, 吴大冰, 王峥嵘.纳米零价铁的制备及应用研究进展[J]. 应用化工,2021,50(3):789-792. doi: 10.3969/j.issn.1671-3206.2021.03.047YAN Z C, WU D B, WANG Z R. Progress of preparation and application of nanoscale zero-valent iron[J]. Applied Chemical Industry,2021,50(3):789-792. doi: 10.3969/j.issn.1671-3206.2021.03.047 [12] DONG H R, DENG J M, XIE Y K, et al. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(Ⅵ) removal from aqueous solution[J]. Journal of Hazardous Materials,2017,332:79-86. doi: 10.1016/j.jhazmat.2017.03.002 [13] SHI L N, ZHANG X, CHEN Z L. Removal of chromium(Ⅵ) from wastewater using bentonite-supported nanoscale zero-valent iron[J]. Water Research,2011,45(2):886-892. doi: 10.1016/j.watres.2010.09.025 [14] BHOWMICK S, CHAKRABORTY S, MONDAL P, et al. Montmorillonite-supported nanoscale zero-valent iron for removal of arsenic from aqueous solution: kinetics and mechanism[J]. Chemical Engineering Journal,2014,243:14-23. doi: 10.1016/j.cej.2013.12.049 [15] ZHU H J, JIA Y F, WU X, et al. Removal of arsenic from water by supported nano zero-valent iron on activated carbon[J]. Journal of Hazardous Materials,2009,172(2/3):1591-1596. [16] 刘美丽, 牛其建, 俞洋洋, 等.碳基材料负载纳米零价铁去除六价铬的研究进展[J]. 环境科学研究,2022,35(3):768-779.LIU M L, NIU Q J, YU Y Y, et al. Progress in removal of hexavalent chromium by carbon-based materials loaded with nano zero-valent iron[J]. Research of Environmental Sciences,2022,35(3):768-779. [17] 庞新宇, 刘文士, 李猛, 等.生物炭环境修复应用研究的文献计量学分析[J]. 环境工程技术学报,2021,11(4):740-749. doi: 10.12153/j.issn.1674-991X.20200261PANG X Y, LIU W S, LI M, et al. Research progress of biochar's application in environmental remediation based on bibliometrics[J]. Journal of Environmental Engineering Technology,2021,11(4):740-749. doi: 10.12153/j.issn.1674-991X.20200261 [18] 黄开友, 申英杰, 王晓岩, 等.生物炭负载纳米零价铁制备及修复六价铬污染土壤技术研究进展[J]. 环境工程,2020,38(11):203-210.HUANG K Y, SHEN Y J, WANG X Y, et al. Review on preparation of bio-carbon loaded nano zero-valent iron and its application in remediating Cr(Ⅵ)-contaminated soil[J]. Environmental Engineering,2020,38(11):203-210. [19] WANG J L, WANG S Z. Preparation, modification and environmental application of biochar: a review[J]. Journal of Cleaner Production,2019,227:1002-1022. doi: 10.1016/j.jclepro.2019.04.282 [20] 邓辉, 李政家, 金志文, 等.棉秆与污泥共热解制备生物炭工艺优化及其结构与吸附性能[J]. 农业工程学报,2016,32(24):248-254. doi: 10.11975/j.issn.1002-6819.2016.24.033DENG H, LI Z J, JIN Z W, et al. Process optimization of char prepared from co-pyrolysis of cotton stalk and sludge and analysis on its structure and adsorption capacity[J]. Transactions of the Chinese Society of Agricultural Engineering,2016,32(24):248-254. doi: 10.11975/j.issn.1002-6819.2016.24.033 [21] ZHANG J, JIN J W, WANG M Y, et al. Co-pyrolysis of sewage sludge and rice husk/bamboo sawdust for biochar with high aromaticity and low metal mobility[J]. Environmental Research,2020,191:110034. doi: 10.1016/j.envres.2020.110034 [22] WANG Z P, WANG J, XIE L K, et al. Influence of the addition of cotton stalk during co-pyrolysis with sewage sludge on the properties, surface characteristics, and ecological risks of biochars[J]. Journal of Thermal Science,2019,28(4):755-762. doi: 10.1007/s11630-019-1100-1 [23] 戴泽军, 李炳堂, 李鹏飞, 等.烟厂污泥热解制备载铁生物炭吸附水中Cr(Ⅵ)的研究[J]. 环境科学与技术,2020,43(11):116-123.DAI Z J, LI B T, LI P F, et al. Preparation of Fe-BC by pyrolysis of tobacco wastewater sludge and its adsorption of Cr(Ⅵ) from aqueous solution[J]. Environmental Science & Technology,2020,43(11):116-123. [24] WANG S S, GAO B, LI Y C, et al. Adsorptive removal of arsenate from aqueous solutions by biochar supported zero-valent iron nanocomposite:batch and continuous flow tests[J]. Journal of Hazardous Materials,2017,322:172-181. [25] QIAN L B, LIU S N, ZHANG W Y, et al. Enhanced reduction and adsorption of hexavalent chromium by palladium and silicon rich biochar supported nanoscale zero-valent iron[J]. Journal of Colloid and Interface Science,2019,533:428-436. doi: 10.1016/j.jcis.2018.08.075 [26] ZHU S S, HO S H, HUANG X C, et al. Magnetic nanoscale zerovalent iron assisted biochar: interfacial chemical behaviors and heavy metals remediation performance[J]. ACS Sustainable Chemistry & Engineering,2017,5(11):9673-9682. [27] SHU Y R, JI B, CUI B H, et al. Almond shell-derived, biochar-supported, nano-zero-valent iron composite for aqueous hexavalent chromium removal: performance and mechanisms[J]. Nanomaterials (Basel, Switzerland),2020,10(2):198. doi: 10.3390/nano10020198 [28] ZHAO L, ZHENG W, MAŠEK O, et al. Roles of phosphoric acid in biochar formation: synchronously improving carbon retention and sorption capacity[J]. Journal of Environmental Quality,2017,46(2):393-401. doi: 10.2134/jeq2016.09.0344 [29] 唐宝玲, 李盟, 陈胜文, 等.生物炭负载零价纳米铁对溶液中的Cr6+去除的研究[J]. 上海第二工业大学学报,2019,36(3):159-165.TANG B L, LI M, CHEN S W, et al. Study on removal of Cr6+ in solution by nano zero-valent iron supported on biochar[J]. Journal of Shanghai Polytechnic University,2019,36(3):159-165. [30] YANG F, ZHANG S S, SUN Y Q, et al. Fabrication and characterization of hydrophilic corn stalk biochar-supported nanoscale zero-valent iron composites for efficient metal removal[J]. Bioresource Technology,2018,265:490-497. doi: 10.1016/j.biortech.2018.06.029 [31] DIAO Z H, DU J J, JIANG D, et al. Insights into the simultaneous removal of Cr6+ and Pb2+ by a novel sewage sludge-derived biochar immobilized nanoscale zero valent iron: coexistence effect and mechanism[J]. Science of the Total Environment,2018,642:505-515. doi: 10.1016/j.scitotenv.2018.06.093 [32] 徐建玲, 张頔, 聂苗青, 等.PEI功能化秸秆生物炭对水中Cr6+的吸附性能[J]. 高等学校化学学报,2020,41(1):155-161. doi: 10.7503/cjcu20190418XU J L, ZHANG D, NIE M Q, et al. Adsorption of Cr6+ on polyethyleneimine-functionalized straw biochar from aqueous solution[J]. Chemical Journal of Chinese Universities,2020,41(1):155-161. doi: 10.7503/cjcu20190418 [33] 侯素珍, 田浩然, 黄超, 等.氨基改性生物炭负载纳米零价铁去除水中Cr(Ⅵ)[J]. 环境科学学报,2020,40(11):3931-3938.HOU S Z, TIAN H R, HUANG C, et al. Removal of Cr(Ⅵ) from aqueous solution by amino-modified biochar supported nano zero-valent iron[J]. Acta Scientiae Circumstantiae,2020,40(11):3931-3938. [34] LIU H K, XU F, XIE Y L, et al. Effect of modified coconut shell biochar on availability of heavy metals and biochemical characteristics of soil in multiple heavy metals contaminated soil[J]. Science of the Total Environment,2018,645:702-709. doi: 10.1016/j.scitotenv.2018.07.115 [35] SAJJADI B, SHRESTHA R M, CHEN W Y, et al. Double-layer magnetized/functionalized biochar composite: role of microporous structure for heavy metal removals[J]. Journal of Water Process Engineering,2021,39:101677. doi: 10.1016/j.jwpe.2020.101677 [36] ZHANG S, LYU H H, TANG J C, et al. A novel biochar supported CMC stabilized nano zero-valent iron composite for hexavalent chromium removal from water[J]. Chemosphere,2019,217:686-694. doi: 10.1016/j.chemosphere.2018.11.040 [37] WU H H, WEI W X, XU C B, et al. Polyethylene glycol-stabilized nano zero-valent iron supported by biochar for highly efficient removal of Cr(Ⅵ)[J]. Ecotoxicology and Environmental Safety,2020,188:109902. doi: 10.1016/j.ecoenv.2019.109902 [38] LI S S, YANG F, LI J S, et al. Porous biochar-nanoscale zero-valent iron composites: synthesis, characterization and application for lead ion removal[J]. Science of the Total Environment,2020,746:141037. doi: 10.1016/j.scitotenv.2020.141037 [39] ZHOU H Y, YE M Y, ZHAO Y K, et al. Sodium citrate and biochar synergistic improvement of nanoscale zero-valent iron composite for the removal of chromium(Ⅵ) in aqueous solutions[J]. Journal of Environmental Sciences,2022,115:227-239. doi: 10.1016/j.jes.2021.05.044 [40] QIAN L B, SHANG X, ZHANG B, et al. Enhanced removal of Cr(Ⅵ) by silicon rich biochar-supported nanoscale zero-valent iron[J]. Chemosphere,2019,215:739-745. doi: 10.1016/j.chemosphere.2018.10.030 [41] 李士凤, 孙洪刚, 姚淑华, 等. 一种生物炭负载纳米铁镍复合材料去除水中六价铬方法: CN110745935A[P]. 2020-02-04. [42] HUANG D L, HU Z X, PENG Z W, et al. Cadmium immobilization in river sediment using stabilized nanoscale zero-valent iron with enhanced transport by polysaccharide coating[J]. Journal of Environmental Management,2018,210:191-200. [43] LIU K, LI F B, CUI J H, et al. Simultaneous removal of Cd(Ⅱ) and As(Ⅲ) by graphene-like biochar-supported zero-valent iron from irrigation waters under aerobic conditions: synergistic effects and mechanisms[J]. Journal of Hazardous Materials,2020,395:122623. doi: 10.1016/j.jhazmat.2020.122623 [44] AMIRI M J, ROOHI R, GIL A. Numerical simulation of Cd(Ⅱ) removal by ostrich bone ash supported nanoscale zero-valent iron in a fixed-bed column system: utilization of unsteady advection-dispersion-adsorption equation[J]. Journal of Water Process Engineering,2018,25:1-14. doi: 10.1016/j.jwpe.2018.05.017 [45] ZHANG Y L, LI Y T, DAI C M, et al. Sequestration of Cd(Ⅱ) with nanoscale zero-valent iron (nZVI): characterization and test in a two-stage system[J]. Chemical Engineering Journal,2014,244:218-226. doi: 10.1016/j.cej.2014.01.061 [46] VENKATESWARLU S, LEE D, YOON M. Bioinspired 2D-carbon flakes and Fe3O4 nanoparticles composite for arsenite removal[J]. ACS Applied Materials & Interfaces,2016,8(36):23876-23885. [47] ZHANG X L, WU M F, DONG H, et al. Simultaneous oxidation and sequestration of As(Ⅲ) from water by using redox polymer-based Fe(Ⅲ) oxide nanocomposite[J]. Environmental Science & Technology,2017,51(11):6326-6334. [48] ZHU Y, ELZINGA E J. Macroscopic and spectroscopic assessment of the cosorption of Fe(Ⅱ) with As(Ⅲ) and As(V) on Al-oxide[J]. Environmental Science & Technology,2015,49(22):13369-13377. [49] BAKSHI S, BANIK C, RATHKE S J, et al. Arsenic sorption on zero-valent iron-biochar complexes[J]. Water Research,2018,137:153-163. doi: 10.1016/j.watres.2018.03.021 [50] ZHOU Y M, GAO B, ZIMMERMAN A R, et al. Biochar-supported zerovalent iron for removal of various contaminants from aqueous solutions[J]. Bioresource Technology,2014,152:538-542. doi: 10.1016/j.biortech.2013.11.021 [51] REYHANITABAR A, ALIDOKHT L, KHATAEE A R, et al. Application of stabilized Fe0 nanoparticles for remediation of Cr(Ⅵ)-spiked soil[J]. European Journal of Soil Science,2012,63(5):724-732. ◇ doi: 10.1111/j.1365-2389.2012.01447.x -




 下载:
下载:


