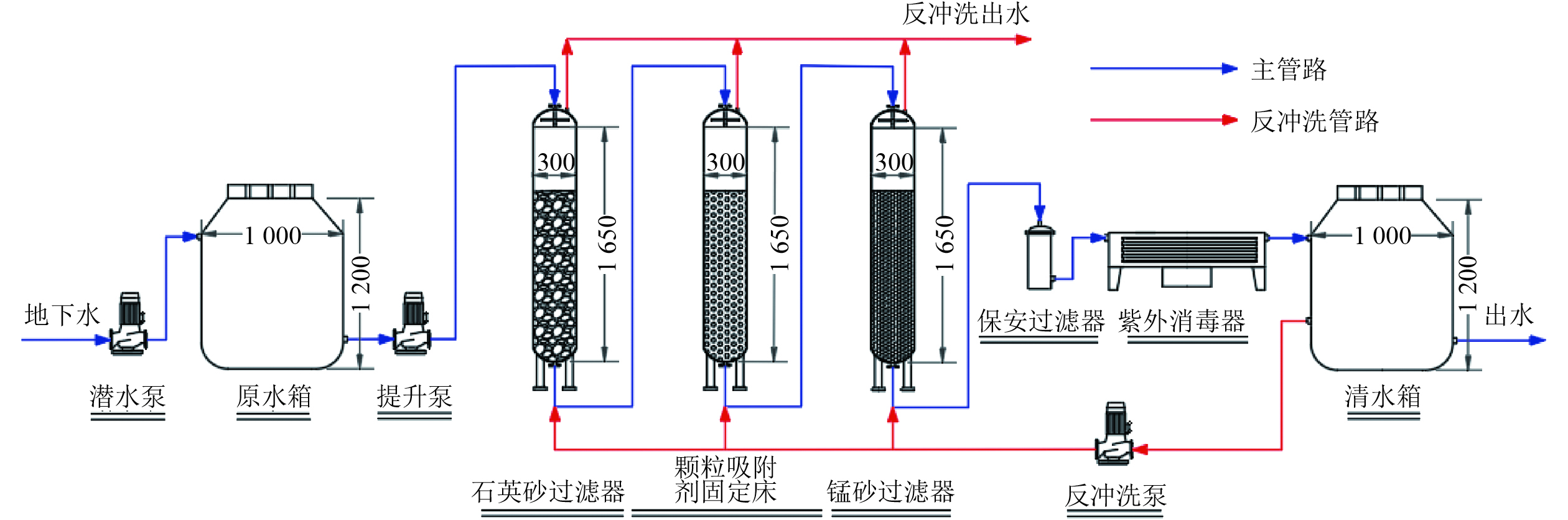
Pilot process of arsenic-containing groundwater purification by iron-based granular adsorbent fixed bed column
-
摘要:
以农村地区的As污染地下水为处理对象,研发了同步去除水中As(Ⅲ)和As(Ⅴ)的铁基颗粒吸附剂(FMGA),设计并建立了以吸附固定床为核心单元的中试除As装置,对As污染地下水的处理效果进行研究。结果表明:在33 d的连续运行过程中,除As装置出水As浓度始终低于GB 5749—2022《生活饮用水卫生标准》规定限值(10 μg/L),吸附固定床首次运行的穿透时间达到786 h;使用0.2 mol/L的NaOH溶液对吸附剂进行原位再生后,吸附固定床再次运行的穿透时间仍可达到750 h,其除As性能的恢复率接近91%;除As装置的出水浊度接近于0,Fe、Mn离子浓度均低于GB 5749—2022的限值(Fe浓度为0.3 mg/L,Mn浓度为0.1 mg/L),FMGA可高效再生回用且无二次污染。吸附动力学表明,FMGA吸附As的过程符合准二级动力学模型,As通过化学吸附被去除;吸附等温线表明,FMGA对As的理论最大吸附容量为74.94 mg/g(pH为7.0)。通过表征研究可知,FMGA最大荷载为89.39 N,具有出色的机械强度。
Abstract:Arsenic (As) pollution in groundwater has become an important environmental issue in China. In order to purify the arsenic-contaminated groundwater in rural areas, an iron-based granular adsorbent (FMGA) capable of synchronously removing As(Ⅲ) and As(Ⅴ) from water was developed and packed into an adsorption fixed bed. A pilot-scale water treatment system was designed and established with a fixed bed as the core unit, which had a good capability for the treatment of As-contaminated groundwater. The results showed that during the continuous operation of 33 days, the residual As concentration in the effluent of the pilot-scale system was continuously below the limit of Standards for Drinking Water Quality (GB 5749-2022 ) (10 μg/L). The breakthrough time of the fixed bed reached 786 h in the first cycle. After the in-situ regeneration using 0.2 mol/L NaOH solution, the breakthrough time of the fixed bed for reuse could still reach 750 h, and the recovery rate of its arsenic adsorption capacity was close to 91%. The turbidity of the effluent for the pilot-scale system was close to zero, and the concentrations of iron and manganese ions were both lower than the limits of the sanitary standard (Fe<0.3 mg/L, Mn<0.1 mg/L). FMGA could be efficiently regenerated and reused without secondary pollution. The adsorption kinetics indicated that the adsorption process of As by FMGA was consistent with the quasi-second-order kinetic model, and As could be removed by chemisorption. The adsorption isotherm showed that the theoretical maximum adsorption capacity of FMGA for As was 74.94 mg/g (pH=7.0). According to the surface characterization results, the maximum load of FMGA was 89.39 N, indicating an excellent mechanical strength.
-
Key words:
- arsenic (As) /
- groundwater /
- fixed bed /
- Fe-Mn composite oxide /
- granular adsorbent /
- pilot-scale experiment
-
表 1 准一级和准二级动力学模型拟合参数
Table 1. The pseudo-first order and pseudo-second order models parameters
准一级动力学模型 准二级动力学模型 qe/(μg/g) k1/h−1 R2 qe/(μg/g) k2/〔g/(μg·h)〕 R2 94.12 0.33 0.979 95.24 4.52 0.999 表 2 颗粒内扩散模型拟合参数
Table 2. Intra-particle diffusion fitting parameters
外扩散阶段 内扩散阶段 吸附反应阶段 kp/〔μg/(g·h1/2)〕 C/(μg/g) R2 kp/〔μg/(g·h1/2)〕 C/(μg/g) R2 kp/〔μg/(g·h1/2)〕 C/(μg/g) R2 2.89 85.64 0.955 6.06 77.25 0.972 1.19 89.95 0.920 表 3 Langmuir和Freundlich模型拟合参数
Table 3. Langmuir and Freundlich model fitting parameters
Freundlich模型 Langmuir模型 KF/〔(mg/g)/(mg/L)n〕 1/n R2 qmax/(mg/g) KL/(L/mg) R2 5.38 1.13 0.991 74.94 0.02 0.987 表 4 除As吸附剂性能比较
Table 4. Comparison of performance of arsenic removal adsorbents
表 5 Thomas模型拟合固定床反应器穿透曲线参数
Table 5. Thomas model fitting fixed bed reactor breakthrough curve parameters
运行周期 t/(min) Q/(L/h) C0/(μg/L) Thomas模型参数 kTh×10−6/〔mL/(min·μg)〕 q0/(mg/g) R2 第一周期 47 160 500 45 7.03 284.8 0.94 第二周期 45 000 500 45 6.44 276.3 0.97 表 6 FMGA与市场中相关的除As吸附剂价格对比
Table 6. Comparison of prices between FMGA and market related As removal adsorbents
吸附剂名称 价格/(元/kg) 生产厂家 FMGA 33.4 本研究 GEH® 80 德国沃驰公司(Watch® Water) FERROLOX-X 80 德国沃驰公司(Watch® Water) TITANSORBTM 120 德国沃驰公司(Watch® Water) KATALOX-LIGHT 40 德国沃驰公司(Watch® Water) Trppsorb & Crystolite 95 德国沃驰公司(Watch® Water) BAYOXIDE® E33 175 德国拜耳公司 -
[1] 汤洁, 卞建民, 李昭阳, 等. 中国饮水型砷中毒区的水化学环境与砷中毒关系[J]. 生态毒理学报,2013,8(2):222-229.TANG J, BIAN J M, LI Z Y, et al. Relationship between hydrochemical environment and arsenism in areas with arsenic poisoning drinking water in China[J]. Asian Journal of Ecotoxicology,2013,8(2):222-229. [2] 国家卫生健康委员会. 生活饮用水卫生标准: GB 5749—2022[S]. 北京: 中国标准出版社, 2022. [3] 梁杜娟, 邱忆南, 吴敏, 等. MgAl-NO3-LDH对水中无机砷、氟的吸附/脱附性能研究[J]. 环境工程技术学报,2016,6(1):22-25.LIANG D J, QIU Y N, WU M, et al. Adsorption of inorganic arsenic As(Ⅲ), As(Ⅴ) and F− on MgAl-NO3-LDH in water[J]. Journal of Environmental Engineering Technology,2016,6(1):22-25. [4] 石乐琪, 郭莉, 吕晨阳, 等. 地下水脱砷技术的研究现状及发展趋势[J]. 环境工程技术学报,2022,12(5):1548-1554.SHI L Q, GUO L, LÜ C Y, et al. Research status and development trend of the technology for arsenic removal from groundwater[J]. Journal of Environmental Engineering Technology,2022,12(5):1548-1554. [5] FEISTEL U, OTTER P, KUNZ S, et al. Field tests of a small pilot plant for the removal of arsenic in groundwater using coagulation and filtering[J]. Journal of Water Process Engineering,2016,14:77-85. doi: 10.1016/j.jwpe.2016.10.006 [6] ORTEGA A, OLIVA I, CONTRERAS K E, et al. Arsenic removal from water by hybrid electro-regenerated anion exchange resin/electrodialysis process[J]. Separation and Purification Technology,2017,184:319-326. doi: 10.1016/j.seppur.2017.04.050 [7] HARFOUSH M, MIRBAGHERI S A, EHTESHAMI M, et al. Arsenic removal from drinking water using low-pressure nanofiltration under various operating conditions[J]. Water Practice and Technology,2018,13(2):295-302. doi: 10.2166/wpt.2018.042 [8] 曾辉平, 于亚萍, 吕赛赛, 等. 基于铁锰泥的除砷颗粒吸附剂制备及其比较[J]. 环境科学,2019,40(11):5002-5008.ZENG H P, YU Y P, LÜ S S, et al. Preparation and comparison of arsenic removal granular adsorbent based on iron-manganese sludge[J]. Environmental Science,2019,40(11):5002-5008. [9] ZHENG Q, HOU J T, HARTLEY W, et al. As(Ⅲ) adsorption on Fe-Mn binary oxides: are Fe and Mn oxides synergistic or antagonistic for arsenic removal[J]. Chemical Engineering Journal,2020,389:124470. doi: 10.1016/j.cej.2020.124470 [10] MOHAPATRA D, MISHRA D, CHAUDHURY G R, et al. Arsenic adsorption mechanism on clay minerals and its dependence on temperature[J]. Korean Journal of Chemical Engineering,2007,24(3):426-430. doi: 10.1007/s11814-007-0073-z [11] HA H T, PHONG P T, MINH T D. Synthesis of iron oxide nanoparticle functionalized activated carbon and its applications in arsenic adsorption[J]. Journal of Analytical Methods in Chemistry,2021,2021:6668490. [12] 唐朝春, 朱蓓, 许荣明, 等. 金属基吸附剂除砷技术研究进展[J]. 环境科学与技术,2020,43(10):221-228.TANG C C, ZHU B, XU R M, et al. Progress in research on arsenic removal technology with metal-based adsorbents[J]. Environmental Science & Technology,2020,43(10):221-228. [13] 龚正, 于琦, 汪娴, 等. 西北地区地下水污染现状及风险调查[J]. 净水技术,2022,41(3):118-133.GONG Z, YU Q, WANG X, et al. Investigation on risks and present situation of groundwater pollution in northwest regions[J]. Water Purification Technology,2022,41(3):118-133. [14] YEO K F H, DONG Y Y, XUE T X, et al. Adsorption of arsenate from groundwater through a fixed bed using iron-coated natural fibres: reusability and sorbent characterisation[J]. International Journal of Environmental Analytical Chemistry, 2023: 1-17. [15] 王黎安. 水凝胶型生物粘合剂的制备和性能研究[D]. 合肥: 中国科学技术大学, 2020. [16] 胡明城. 粒状活性炭固定床吸附系统的重要设计参数: 空床接触时间(EBCT)[J]. 化工给排水设计,1998,29(3):17-18.HU M C. An important design parameter of granular activated carbon fixed bed adsorption system: empty bed contact time (EBCT)[J]. Industrial Water & Wastewater,1998,29(3):17-18. [17] 张艳素, 豆小敏, 于新, 等. 锆铁复合氧化物颗粒对As(Ⅴ)的去除研究[J]. 环境化学,2011,30(8):1396-1404.ZHANG Y S, DOU X M, YU X, et al. Arsenic(Ⅴ) removal with a granular zirconium-iron oxide adsorbent[J]. Environmental Chemistry,2011,30(8):1396-1404. [18] 李海宁. 铁锰复合氧化物/壳聚糖珠吸附材料制备及其去除水中砷、磷的研究[D]. 烟台: 烟台大学, 2016. [19] CHANG F F, QU J H, ZHAO X, et al. Migration of manganese and iron during the adsorption-regeneration cycles for arsenic removal[J]. Frontiers of Environmental Science & Engineering in China,2011,5(4):512-518. [20] CHEN J, WANG J Y, ZHANG G S, et al. Facile fabrication of nanostructured cerium-manganese binary oxide for enhanced arsenite removal from water[J]. Chemical Engineering Journal,2018,334:1518-1526. doi: 10.1016/j.cej.2017.11.062 [21] ZHANG G S, FAN F, LI X P, et al. Superior adsorption of thallium(Ⅰ) on titanium peroxide: performance and mechanism[J]. Chemical Engineering Journal,2018,331:471-479. doi: 10.1016/j.cej.2017.08.053 [22] 王建燕, 张传巧, 陈静, 等. 新型铁铜锰复合氧化物颗粒吸附剂As(Ⅲ)吸附行为与机制研究[J]. 环境科学学报,2019,39(8):2575-2585.WANG J Y, ZHANG C Q, CHEN J, et al. The adsorption of As(Ⅲ) on a novel granular Fe-Cu-Mn trimetal oxide (GFCM): behavior and mechanism[J]. Acta Scientiae Circumstantiae,2019,39(8):2575-2585. [23] PINTOR A M A, VIEIRA B R C, SANTOS S C R, et al. Arsenate and arsenite adsorption onto iron-coated cork granulates[J]. Science of the Total Environment,2018,642:1075-1089. doi: 10.1016/j.scitotenv.2018.06.170 [24] ZENG H P, WANG F S, XU K, et al. Preparation of manganese sludge strengthened chitosan-alginate hybrid adsorbent and its potential for As(Ⅲ) removal[J]. International Journal of Biological Macromolecules,2020,149:1222-1231. doi: 10.1016/j.ijbiomac.2020.02.030 [25] ZENG H P, YU Y P, WANG F S, et al. Arsenic(Ⅴ) removal by granular adsorbents made from water treatment residuals materials and chitosan[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects,2020,585:124036. doi: 10.1016/j.colsurfa.2019.124036 [26] ZENG H P, SUN S Q, XU K, et al. Adsorption of As(Ⅴ) by magnetic alginate-chitosan porous beads based on iron sludge[J]. Journal of Cleaner Production,2022,359:132117. doi: 10.1016/j.jclepro.2022.132117 [27] ZENG H P, XU K, WANG F S, et al. Adsorption of As(Ⅲ) from aqueous solutions using MnO2 strengthened WTRs-chitosan beads made by homogenous method with freeze-drying[J]. Reactive and Functional Polymers,2021,167:105016. doi: 10.1016/j.reactfunctpolym.2021.105016 [28] 常冰. 新型铝基颗粒去除水中砷、氟的效能及机理研究[D]. 杨凌: 西北农林科技大学, 2016. [29] 叶树芯. 铁锰复合氧化物新型固定化及其除砷特性研究[D]. 武汉: 华中农业大学, 2016. [30] GUO X J, CHEN F H. Removal of arsenic by bead cellulose loaded with iron oxyhydroxide from groundwater[J]. Environmental Science & Technology,2005,39(17):6808-6818. [31] CHEN S H, YUE Q Y, GAO B Y, et al. Adsorption of hexavalent chromium from aqueous solution by modified corn stalk: a fixed-bed column study[J]. Bioresource Technology,2012,113:114-120. doi: 10.1016/j.biortech.2011.11.110 [32] 李圭白, 梁恒, 余华荣, 等. 锰质活性滤膜化学催化氧化除锰机理研究[J]. 给水排水,2019,55(5):6-10. doi: 10.13789/j.cnki.wwe1964.2019.05.001LI G B, LIANG H, YU H R, et al. Research on manganese removal by chemical auto-catalytic oxidation mechanism involved in active manganese oxides film[J]. Water & Wastewater Engineering,2019,55(5):6-10. doi: 10.13789/j.cnki.wwe1964.2019.05.001 [33] 仲琳. 锰砂对地下水除锰的化学作用与生物作用效果研究[D]. 哈尔滨: 哈尔滨工业大学, 2019. [34] LIU Y Q, CAI L K, WANG X Y, et al. Efficient adsorption of arsenic in groundwater by hydrated iron oxide and ferromanganese oxide chitosan gel beads[J]. Separation and Purification Technology,2023,315:123692. ◇ doi: 10.1016/j.seppur.2023.123692 -





 下载:
下载: