Removal efficiency of 2,4-dichlorophenol by persulfate activated with ball-milling vanadium-titanium magnetite tailings
-
摘要:
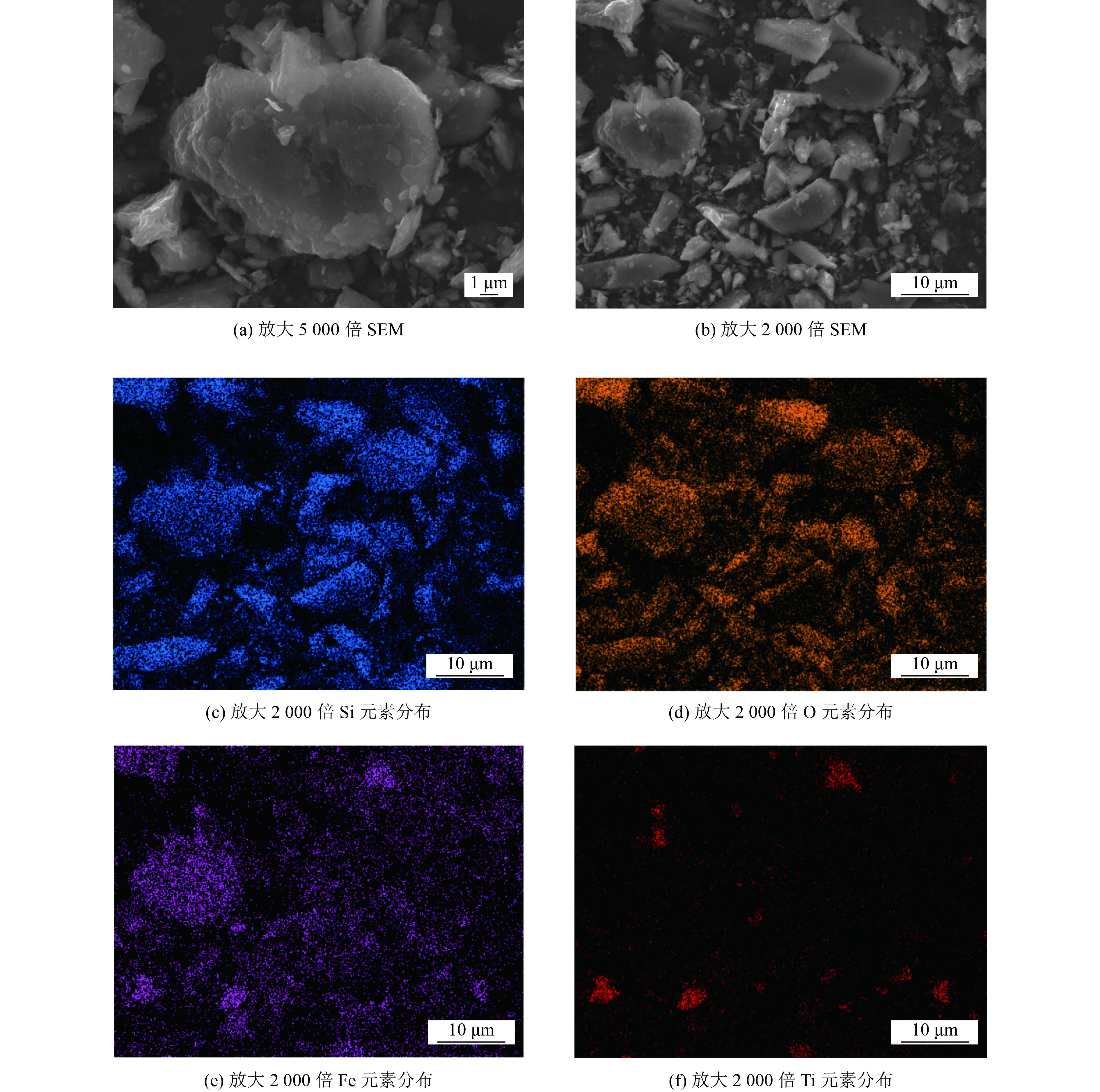
为探究球磨钒钛磁铁矿尾矿(B-VTMT)活化过硫酸盐(PS)去除地下水中有机污染物2,4-二氯苯酚(2,4-DCP)可行性,采用扫描电子显微镜、X射线衍射仪、X射线光电子能谱等表征手段对B-VTMT的形貌和组成进行测试分析,探讨B-VTMT投加量、PS初始浓度、初始pH、2,4-DCP初始浓度及地下水常见阴离子(Cl–、NO3 –、SO4 2–)对2,4-DCP去除率的影响。结果表明:在B-VTMT投加量为0.5 g/L,PS浓度为5 mmol/L,2,4-DCP初始浓度为20 mg/L,初始pH为7.1,室温条件下39 h内2,4-DCP的去除率为45.4%。自由基淬灭和捕获试验证实,硫酸根自由基(SO4 –·)和羟基自由基(·OH)是体系去除2,4-DCP的主要活性自由基,通过高效液相色谱-质谱仪(HPLC-MS)识别了8种中间产物,推测了2,4-DCP可能的降解路径。地下水中Cl–提高了2,4-DCP的去除率,而NO3 –和SO4 2–降低了2,4-DCP的去除率。研究显示,B-VTMT作为PS的活化剂是一种很有前景的尾矿资源化利用方式。
Abstract:The removal feasibility of 2,4-dichlorophenol (2,4-DCP) in groundwater by persulfate (PS) activated with ball-milling vanadium-titanium magnetite tailings (B-VTMT) was investigated. The morphology and composition of B-VTMT were analyzed by scanning electron microscope, X-ray diffraction, and X-ray photoelectron spectroscopy. The effects of B-VTMT dosages, initial PS concentrations, initial pH, initial 2,4-DCP concentrations, and common anions in groundwater (Cl–, NO3 –, and SO4 2–) on 2,4-DCP removal efficiency were explored. The experiment results indicated that: The removal efficiency of 2,4-DCP was 45.4% within 39 h at room temperature under the conditions of B-VTMT dosage of 0.5 g/L, initial PS concentration of 5 mmol/L, initial 2,4-DCP concentration of 20 mg/L and initial pH 7.1. Radical quenching experiments and electron spin-resonance (ESR) spectroscopy confirmed that sulfate radical (SO4 –·) and hydroxyl radical (·OH) were the main free radicals of 2,4-DCP removal. Eight intermediates were identified by high performance liquid chromatography-mass spectrometry (HPLC-MS). The possible degradation pathways of 2,4-DCP were speculated. The presence of chloride ions promoted the 2,4-DCP removal efficiency, while nitrate ions and sulfate ions inhibited the 2,4-DCP removal efficiency. It was concluded that B-VTMT could effectively activate PS to remove 2,4-DCP in groundwater, which was a promising way of tailings resource utilization.
-
表 1 VTMT主要化学成分
Table 1. Main chemical components of VTMT
% 成分 含量 成分 含量 SiO2 41.63 Al2O3 10.98 Fe 14.80 MnO 0.21 CaO 13.16 V2O5 0.11 TiO2 5.05 烧失量 2.07 MgO 9.94 表 2 反应前后体系中铁、锰、钛和钒金属离子浓度
Table 2. Iron, manganese, titanium and vanadium ion concentrations before and after reaction
μg/L 金属离子 反应前 反应后 Fe 26.28 967.54 Mn 0.40 1.04 Ti 5.13 112.44 V 未检出 未检出 -
[1] PERSSON Y, SHCHUKAREV A, OBERG L, et al. Dioxins, chlorophenols and other chlorinated organic pollutants in colloidal and water fractions of groundwater from a contaminated sawmill site[J]. Environmental Science and Pollution Research International,2008,15(6):463-471. doi: 10.1007/s11356-008-0014-3 [2] LI Y T, YUE D, WANG B, et al. Degradation of MDEA in aqueous solution in the thermally activated persulfate system[J]. Environmental Technology,2017,38(6):730-736. doi: 10.1080/09593330.2016.1210239 [3] MORADI M, GHANBARI F, MANSHOURI M, et al. Photocatalytic degradation of azo dye using nano-ZrO2/UV/Persulfate: response surface modeling and optimization[J]. Korean Journal of Chemical Engineering,2016,33(2):539-546. doi: 10.1007/s11814-015-0160-5 [4] 周金倩, 马建立, 商晓甫, 等.过硫酸盐氧化修复多环芳烃污染土壤的研究[J]. 环境工程技术学报,2020,10(3):482-486. doi: 10.12153/j.issn.1674-991X.20190148ZHOU J Q, MA J L, SHANG X F, et al. Persulfate oxidation for remediation of polycyclic aromatic hydrocarbon contaminated soil[J]. Journal of Environmental Engineering Technology,2020,10(3):482-486. doi: 10.12153/j.issn.1674-991X.20190148 [5] SUN D D, YAN X X, XUE W P. Oxidative degradation of dimethyl phthalate (DMP) by persulfate catalyzed by Ag+ combined with microwave irradiation[J]. Advanced Materials Research,2012,610:1209-1212. [6] KANG J, WU W C, LIU W X, et al. Zero-valent iron (ZVI) activation of persulfate (PS) for degradation of Para-chloronitrobenzene in soil[J]. Bulletin of Environmental Contamination and Toxicology,2019,103(1):140-146. doi: 10.1007/s00128-018-2511-5 [7] 卢成龙, 常红, 孙福红.环境水体中自由基的检测技术研究进展[J]. 环境工程技术学报,2022,12(1):70-80. doi: 10.12153/j.issn.1674-991X.20210322LU C L, CHANG H, SUN F H. Progress on the detection technology of free radicals in waters[J]. Journal of Environmental Engineering Technology,2022,12(1):70-80. doi: 10.12153/j.issn.1674-991X.20210322 [8] 许若梦, 吴桐, 锁瑞娟, 等.基于不同自由基的高级氧化技术对水中诺氟沙星的去除效果[J]. 环境工程技术学报,2020,10(3):433-439. doi: 10.12153/j.issn.1674-991X.20190177XU R M, WU T, SUO R J, et al. Removal performance of norfloxacin from waters by advanced oxidation processes based on different free radicals[J]. Journal of Environmental Engineering Technology,2020,10(3):433-439. doi: 10.12153/j.issn.1674-991X.20190177 [9] 程莹, 臧纪, 宋骏杰, 等.基于臭氧微纳米气泡的O3-H2O2体系降解有机污染物的效能与影响因素[J]. 环境工程技术学报,2022,12(4):1317-1323. doi: 10.12153/j.issn.1674-991X.20220194CHENG Y, ZANG J, SONG J J, et al. Degradation efficiency and influencing factors of organic contaminants in O3-H2O2 system based on ozone micro-nanobubbles[J]. Journal of Environmental Engineering Technology,2022,12(4):1317-1323. doi: 10.12153/j.issn.1674-991X.20220194 [10] TEEL A L, AHMAD M, WATTS R J. Persulfate activation by naturally occurring trace minerals[J]. Journal of Hazardous Materials,2011,196:153-159. doi: 10.1016/j.jhazmat.2011.09.011 [11] LIU H Z, BRUTON T A, DOYLE F M, et al. In situ chemical oxidation of contaminated groundwater by persulfate: decomposition by Fe(Ⅲ)- and Mn(Ⅳ)-containing oxides and aquifer materials[J]. Environmental Science & Technology,2014,48(17):10330-10336. [12] KERMANI M, MOHAMMADI F, KAKAVANDI B, et al. Simultaneous catalytic degradation of 2, 4-D and MCPA herbicides using sulfate radical-based heterogeneous oxidation over persulfate activated by natural hematite (α-Fe2O3/PS)[J]. Journal of Physics and Chemistry of Solids,2018,117:49-59. doi: 10.1016/j.jpcs.2018.02.009 [13] LAI L D, ZHOU H Y, ZHANG H, et al. Activation of peroxydisulfate by natural titanomagnetite for atrazine removal via free radicals and high-valent iron-oxo species[J]. Chemical Engineering Journal,2020,387:124165. doi: 10.1016/j.cej.2020.124165 [14] LAI L D, JI H D, ZHANG H, et al. Activation of peroxydisulfate by V-Fe concentrate ore for enhanced degradation of carbamazepine: surface ≡V(Ⅲ) and ≡V(Ⅳ) as electron donors promoted the regeneration of ≡Fe(Ⅱ)[J]. Applied Catalysis B:Environmental,2021,282:119559. doi: 10.1016/j.apcatb.2020.119559 [15] 范敦城, 倪文, 李瑾, 等.铁尾矿再选粗精矿深度还原含铁硅酸盐矿物的生成与还原[J]. 中南大学学报(自然科学版),2015,46(6):1973-1980.FAN D C, NI W, LI J, et al. Generation and reduction mechanism of silicate minerals containing iron in deep reduction of rough concentrate from iron tailings[J]. Journal of Central South University (Science and Technology),2015,46(6):1973-1980. [16] 叶倩, 王城晨, 王明新, 等.球磨硫铁矿-过硫酸盐联合降解水中硝基苯和苯胺[J]. 环境工程学报,2021,15(01):30-42. doi: 10.12030/j.cjee.202005099YE Q, WANG C C, WANG M X, et al. Degradation of nitrobenzene and aniline in water by ball milling pyrite and persulfate[J]. Chinese Journal of Environmental Engineering,2021,15(01):30-42. doi: 10.12030/j.cjee.202005099 [17] DU M M, ZHANG Y Q, ZENG X L, et al. Enhancement of ball-miling on pyrite/zero-valent iron for arsenic removal in water: a mechanistic study[J]. Chemosphere,2020,249:126130. doi: 10.1016/j.chemosphere.2020.126130 [18] ZHOU T, ZOU X L, MAO J, et al. Decomposition of sulfadiazine in a sonochemical Fe0-catalyzed persulfate system: parameters optimizing and interferences of wastewater matrix[J]. Applied Catalysis B:Environmental,2016,185:31-41. doi: 10.1016/j.apcatb.2015.12.004 [19] LAI L D, ZHOU H Y, LAI B. Heterogeneous degradation of bisphenol A by peroxymonosulfate activated with vanadium-titanium magnetite: Performance, transformation pathways and mechanism[J]. Chemical Engineering Journal,2018,349:633-645. doi: 10.1016/j.cej.2018.05.134 [20] DONG H R, HOU K J, QIAO W W, et al. Insights into enhanced removal of TCE utilizing sulfide-modified nanoscale zero-valent iron activated persulfate[J]. Chemical Engineering Journal,2019,359:1046-1055. doi: 10.1016/j.cej.2018.11.080 [21] ANIPSITAKIS G P, STATHATOS E, DIONYSIOU D D. Heterogeneous activation of oxone using Co3O4[J]. The Journal of Physical Chemistry B,2005,109(27):13052-13055. doi: 10.1021/jp052166y [22] CHERIFI Y, ADDAD A, VEZIN H, et al. PMS activation using reduced graphene oxide under sonication: efficient metal-free catalytic system for the degradation of rhodamine B, bisphenol A, and tetracycline[J]. Ultrasonics Sonochemistry,2019,52:164-175. doi: 10.1016/j.ultsonch.2018.11.012 [23] MA Q L, ZHANG X Y, GUO R N, et al. Persulfate activation by magnetic γ-Fe2O3/Mn3O4 nanocomposites for degradation of organic pollutants[J]. Separation and Purification Technology,2019,210:335-342. doi: 10.1016/j.seppur.2018.06.060 [24] ZHANG P, SONG D B, XUEJINGXU, et al. Sulfidated zero valent iron as a persulfate activator for oxidizing organophosphorus pesticides (OPPs) in aqueous solution and aged contaminated soil columns[J]. Chemosphere,2021,281:130760. doi: 10.1016/j.chemosphere.2021.130760 [25] YAMASHITA T, HAYES P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials[J]. Applied Surface Science,2008,254(8):2441-2449. doi: 10.1016/j.apsusc.2007.09.063 [26] LI H X, WAN J Q, MA Y W, et al. New insights into the role of zero-valent iron surface oxidation layers in persulfate oxidation of dibutyl phthalate solutions[J]. Chemical Engineering Journal,2014,250:137-147. doi: 10.1016/j.cej.2014.03.092 [27] XU L J, WANG J L. Magnetic nanoscaled Fe3O4/CeO2 composite as an efficient Fenton-like heterogeneous catalyst for degradation of 4-chlorophenol[J]. Environmental Science & Technology,2012,46(18):10145-10153. [28] WANG Y Y, WANG L, ZHANG Y L, et al. Perdisulfate-assisted advanced oxidation of 2, 4-dichlorophenol by bio-inspired iron encapsulated biochar catalyst[J]. Journal of Colloid and Interface Science,2021,592:358-370. doi: 10.1016/j.jcis.2021.02.056 [29] WU Z H, FANG J Y, XIANG Y Y, et al. Roles of reactive chlorine species in trimethoprim degradation in the UV/chlorine process: kinetics and transformation pathways[J]. Water Research,2016,104:272-282. doi: 10.1016/j.watres.2016.08.011 [30] 王慧, 李晓东, 张玉秀, 等.氯离子影响热活化过硫酸盐降解苯酚的机理研究[J]. 环境科学研究,2023,36(1):150-158. doi: 10.13198/j.issn.1001-6929.2022.08.06WANG H, LI X D, ZHANG Y X, et al. Mechanisms on the impacts of chloride ions on the degradation of phenol by thermally activated persulfate[J]. Research of Environmental Sciences,2023,36(1):150-158. doi: 10.13198/j.issn.1001-6929.2022.08.06 [31] MA W J, DU Y C, WANG N, et al. ZIF-8 derived nitrogen-doped porous carbon as metal-free catalyst of peroxymonosulfate activation[J]. Environmental Science and Pollution Research International,2017,24(19):16276-16288. doi: 10.1007/s11356-017-9191-2 [32] RAYAROTH M P, LEE C S, ARAVIND U K, et al. Oxidative degradation of benzoic acid using Fe0- and sulfidized Fe0-activated persulfate: a comparative study[J]. Chemical Engineering Journal,2017,315:426-436. doi: 10.1016/j.cej.2017.01.031 [33] SUGIMOTO T, WANG Y S. Mechanism of the shape and structure control of monodispersed α-Fe2O3 particles by sulfate ions[J]. Journal of Colloid and Interface Science,1998,207(1):137-149. doi: 10.1006/jcis.1998.5741 [34] SIEDLECKA E M, WIĘCKOWSKA A, STEPNOWSKI P. Influence of inorganic ions on MTBE degradation by Fenton's reagent[J]. Journal of Hazardous Materials,2007,147(1/2):497-502. [35] DEVI L G, MUNIKRISHNAPPA C, NAGARAJ B, et al. Effect of chloride and sulfate ions on the advanced photo Fenton and modified photo Fenton degradation process of Alizarin Red S[J]. Journal of Molecular Catalysis A:Chemical,2013,374:125-131. [36] WU X L, GU X G, LU S G, et al. Strong enhancement of trichloroethylene degradation in ferrous ion activated persulfate system by promoting ferric and ferrous ion cycles with hydroxylamine[J]. Separation and Purification Technology,2015,147:186-193. ⊕ doi: 10.1016/j.seppur.2015.04.031 -





 下载:
下载: