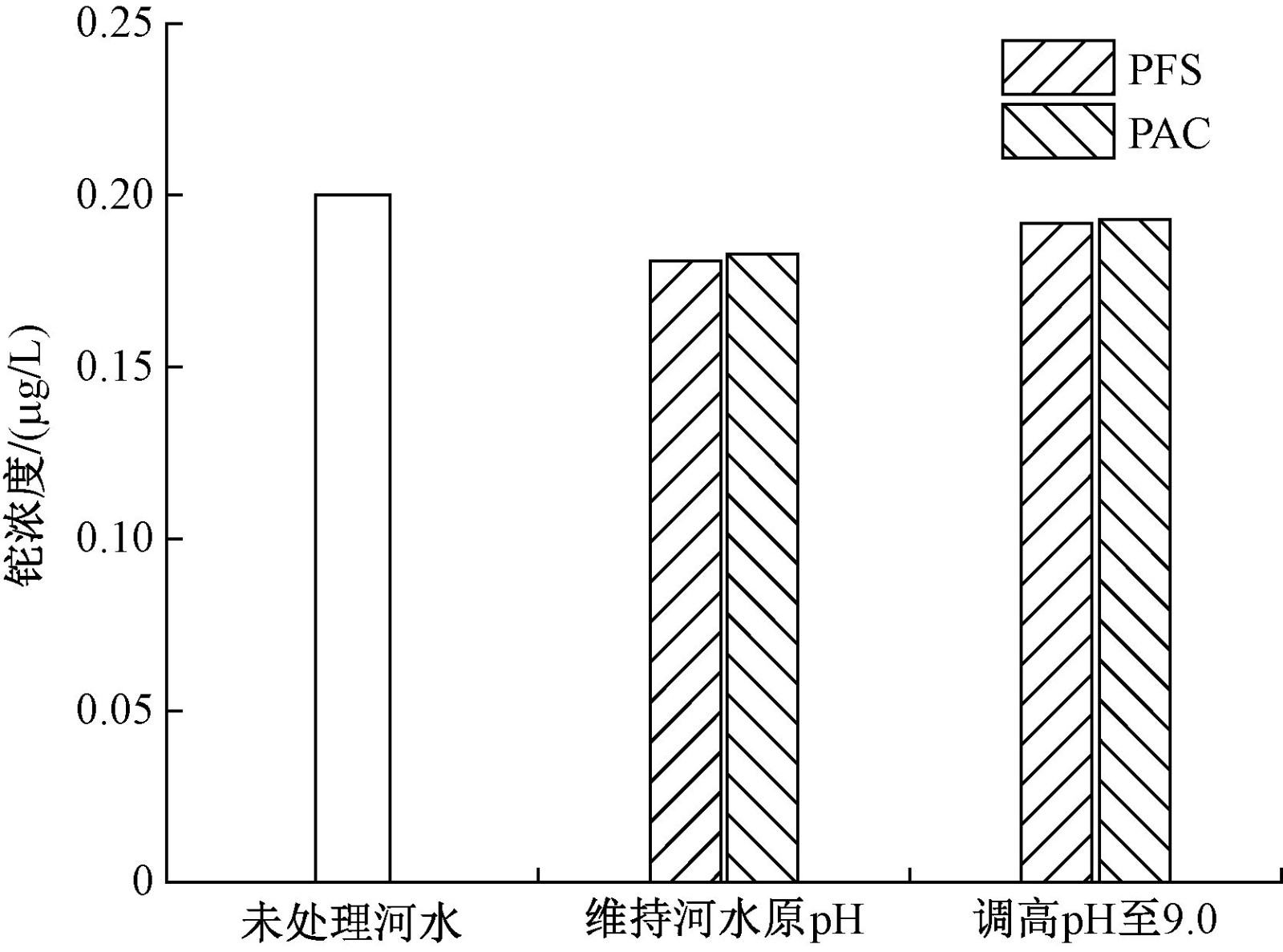
| [1] |
LIN T S, NRIAGU J. Thallium speciation in the great lakes[J]. Environmental Science & Technology,1999,33(19):3394-3397.
|
| [2] |
US EPA. Toxic and priority pollutants under the Clean Water Act[EB/OL]. https://www.epa.gov/eg/toxic-and-priority-pollutants-under-clean-water-act.
|
| [3] |
CVJETKO P, CVJETKO I, PAVLICA M. Thallium toxicity in humans[J]. Arhiv Za Higijenu Rada i Toksikologiju,2010,61(1):111-119. doi: 10.2478/10004-1254-61-2010-1976
|
| [4] |
生态环境部. 优先控制化学品名录(第二批)[A/OL]. [2021-07-20]. http://www.mee.gov.cn/xxgk2018/xxgk/xxgk01/202011/W020201113571806102775.pdf.
|
| [5] |
张红英, 陈永亨.铊的环境污染与迁移转化[J]. 广东微量元素科学,2000,7(10):1-6. doi: 10.3969/j.issn.1006-446X.2000.10.001ZHANG H Y, CHEN Y H. A review of thallium pollution migration and transformation[J]. Trace Elements Science,2000,7(10):1-6. doi: 10.3969/j.issn.1006-446X.2000.10.001
|
| [6] |
生态环境部. 关于发布《电子工业水污染物排放标准》等8项标准(含标准修改单)的公告[A/OL]. [2021-07-20]. http://www.mee.gov.cn/xxgk2018/xxgk/xxgk01/202012/t20201223_814469.html.
|
| [7] |
陈永亨, 张平, 吴颖娟, 等.广东北江铊污染的产生原因与污染控制对策[J]. 广州大学学报(自然科学版),2013,12(4):26-31.CHEN Y H, ZHANG P, WU Y J, et al. The reasons and the control technology for thallium pollution in Beijiang, Guangdong Province[J]. Journal of Guangzhou University (Natural Science Edition),2013,12(4):26-31.
|
| [8] |
余素华, 巢猛, 胡小芳.水源突发性铊污染的去除试验研究[J]. 城镇供水,2014(3):78-79. doi: 10.3969/j.issn.1002-8420.2014.03.023
|
| [9] |
胡小芳, 巢猛.水源水中金属铊污染的应急处理研究[J]. 城镇供水,2016(4):73-77. doi: 10.3969/j.issn.1002-8420.2016.04.016
|
| [10] |
梁国华, 蔡展航, 刘永志, 等.原水突发性铊污染应急处理研究[J]. 科技资讯,2014,12(11):120-124. doi: 10.3969/j.issn.1672-3791.2014.11.078LIANG G H, CAI Z H, LIU Y Z, et al. Research on the emergency treatment of raw water accidentlally polluted by thallium[J]. Science & Technology Information,2014,12(11):120-124. doi: 10.3969/j.issn.1672-3791.2014.11.078
|
| [11] |
HUANGFU X L, JIANG J, LU X X, et al. Adsorption and oxidation of thallium(Ⅰ) by a nanosized manganese dioxide[J]. Water, Air, & Soil Pollution,2014,226(1):1-9.
|
| [12] |
HUANGFU X L, JIANG J, MA J, et al. Aggregation kinetics of manganese dioxide colloids in aqueous solution: influence of humic substances and biomacromolecules[J]. Environmental Science & Technology,2013,47(18):10285-10292.
|
| [13] |
WILLIAMS-BEAM C, TWIDWELL L G. Removal of thallium from wastewater[M/OL]. [2021-07-20]. https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118804407.ch48.
|
| [14] |
卢然, 王夏晖, 伍思扬, 等. 我国铅锌冶炼工业废水铊污染状况与处理技术[J]. 环境工程技术学报, 2021, 11(4): 763-768.LU R, WANG X H, WU S Y, et al. Thallium pollution status and treatment technology of wastewater from lead-zinc smelting industry in China[J]. Journal of Environmental Engineering Technology, 2021, 11(4): 763-768.
|
| [15] |
XU H Y, LUO Y L, WANG P, et al. Removal of thallium in water/wastewater: a review[J]. Water Research,2019,165:114981. doi: 10.1016/j.watres.2019.114981
|
| [16] |
ZOU Y J, CHENG H J, WANG H N, et al. Thallium(Ⅰ) oxidation by permanganate and chlorine: kinetics and manganese dioxide catalysis[J]. Environmental Science & Technology,2020,54(12):7205-7216.
|
| [17] |
LIU J, LUO X W, SUN Y Q, et al. Thallium pollution in China and removal technologies for waters: a review[J]. Environment International,2019,126:771-790. doi: 10.1016/j.envint.2019.01.076
|
| [18] |
LIN T S, NRIAGU J. Revised hydrolysis constants for thallium(Ⅰ) and thallium(Ⅲ) and the environmental implications[J]. Journal of the Air & Waste Management Association,1998,48(2):151-156.
|
| [19] |
WAN S L, MA M H, LÜ L, et al. Selective capture of thallium(Ⅰ) ion from aqueous solutions by amorphous hydrous manganese dioxide[J]. Chemical Engineering Journal,2014,239:200-206. doi: 10.1016/j.cej.2013.11.010
|
| [20] |
朱保虎, 陈小敏, 杨文, 等.改性沸石对水中微量汞的吸附性能[J]. 环境工程学报,2016,10(11):6261-6268. doi: 10.12030/j.cjee.201507019ZHU B H, CHEN X M, YANG W, et al. Characterization of trace mercury adsorption by modified zeolite[J]. Chinese Journal of Environmental Engineering,2016,10(11):6261-6268. doi: 10.12030/j.cjee.201507019
|
| [21] |
张良静, 尚长健, 韩旭, 等. 锰氧化物对水体中铊的去除机制研究进展[J]. 环境科学研究, 2021, 34(6): 1387-1396.ZHANG L J, SHANG C J, HAN X, et al. Mechanisms of thallium removal from water by manganese oxides: a review[J]. Research of Environmental Sciences, 2021, 34(6): 1387-1396.
|
| [22] |
易文杰, 刘妍妍, 林朋飞.水源重金属污染的供水应急处理技术研究[J]. 矿业研究与开发,2020,40(8):81-85.YI W J, LIU Y Y, LIN P F. Study on emergency treatment technology of water supply under the heavy metal pollution of water source[J]. Mining Research and Development,2020,40(8):81-85.
|
| [23] |
LI H S, ZHANG H G, LONG J Y, et al. Combined Fenton process and sulfide precipitation for removal of heavy metals from industrial wastewater: bench and pilot scale studies focusing on in-depth thallium removal[J]. Frontiers of Environmental Science & Engineering,2019,13(4):1-12.
|
| [24] |
BRODERIUS S J, SMITH L L. Effect of hydrogen sulfide on fish and invertebrates: Part Ⅱ. hydrogen sulfide determination and relationship between pH and sulfide toxicity. final report[R/OL]. [2021-06-20]. https://www.osti.gov/biblio/7292199.
|
| [25] |
DIMITROV R I, BOYANOV B S. Oxidation of metal sulphides and determination of characteristic temperatures by DTA and TG[J]. Journal of Thermal Analysis and Calorimetry,2000,61(1):181-189. ◇ doi: 10.1023/A:1010181112713
|





 下载:
下载: